Larval Host Plant Selection and Daily Behavior of Poweshiek Skipperling
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Threatened & Endangered Species
Threatened & Endangered Species Iowa Animal ID Guide September 2011 Amphibians Butterflies Fresh Water Mussels Mammals Birds Fish Land Snails Reptiles A special thanks to the Iowa Department of Natural Resources for providing content to this guide. Natural Resources Conservation Service Helping People Help the Land www.ia.nrcs.usda.gov USDA is an equal opportunity provider and employer. How to use the Threatened & Endangered Species Iowa Animal ID Guide: • Endangered species are fish, plant life, or wildlife in danger of extinction throughout all or a significant part of its range. • Threatened species likely become endangered within the foreseeable future throughout all or a significant part of its range. • Orange color-coded species are Iowa’s endangered animal species. They are listed in alphabetical order by common name. • Blue color-coded species are Iowa’s threatened animal species. They are also listed in alphabetical order by common name. • The scientific name for each species is listed below the common name. • Maps on each page highlight the species range in Iowa. Counties filled with a lighter color are only federally protected, while those with a darker color are both state and federally protected. Categories for each species: Amphibians Fish Mammals Birds Fresh Water Mussels Butterflies Land Snails Reptiles Endangered Animal Species Barn owl Tyto alba Habitat Nests and roosts in dark, secluded places. Often found in old barns and abandoned buildings. Barn owls hunt in grassland habitats along field edges, fence rows, and wetland edges where pray is most available. Appropriate practices • Establish grassland to attract prey (200 acres adjacent to potential barn nesting sites can produce good results). -

Specimen Records of Oarisma Scudder 1872 (Lepidoptera: Hesperiidae) in the Oregon State Arthropod Collection, OSU, Corvallis OR
Catalog: Oregon State Arthropod Collection Vol 1(1), 1-3 Specimen records of Oarisma Scudder 1872 (Lepidoptera: Hesperiidae) in the Oregon State Arthropod Collection, OSU, Corvallis OR Jon H. Shepard Christopher J. Marshall Oregon State Arthropod Collection, Department of Integrative Biology, Oregon State University, Corvallis OR 97331 Abstract: A dataset of 260 skipperling specimens belonging to the genus Oarisma is presented along with information pertaining to how the label data was digitized and the metadata standards adopted. A brief synopsis of the dataset is provided along with a supplemental .csv file containing the data records themselves. Cite this work, including the attached dataset, as: Shepard, J. H, C. J. Marshall. 2017. Specimen records of Oarisma Scudder 1872 (Lepidoptera: Hesperiidae) in the Oregon State Arthropod Collection, OSU, Corvallis, OR.. Catalog: Oregon State Arthropod Collection 1(1) p.1-3. http://doi.org/10.5399/osu/Cat_OSAC.1.1.3995 Introduction In 2016, as part of LepNet (Seltmann et. al., 2017) - a national effort to create digital records for North American Lepidoptera - the Oregon State Arthropod Collection began digitizing label data associated with its butterfly and moth collection. While most of the Hesperiidae has not yet been digitally captured a concerted effort was launched across the LepNet participants to see if we could rapidly generate a dataset for Oarisma with the aim of assisting conservation efforts forOarisma poweshiek (Parker, 1870; Fig 1) being led by Dr. Anna Monfils at Central Michigan University. The dataset published herein contains the label data for all specimens residing at the Oregon State Arthropod Collection as of August 2017. -
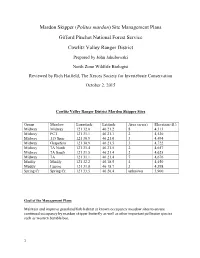
Mardon Skipper Site Management Plans
Mardon Skipper (Polites mardon) Site Management Plans Gifford Pinchot National Forest Service Cowlitz Valley Ranger District Prepared by John Jakubowski North Zone Wildlife Biologist Reviewed by Rich Hatfield, The Xerces Society for Invertebrate Conservation October 2, 2015 Cowlitz Valley Ranger District Mardon Skipper Sites Group Meadow Longitude Latitude Area (acres) Elevation (ft.) Midway Midway 121 32.0 46 21.2 8 4,313 Midway PCT 121 31.1 46 21.1 2 4,530 Midway 115 Spur 121 30.9 46 21.0 3 4,494 Midway Grapefern 121 30.9 46 21.5 3 4,722 Midway 7A North 121 31.4 46 21.5 2 4,657 Midway 7A South 121 31.5 46 21.4 2 4,625 Midway 7A 121 31.1 46 21.4 7 4,676 Muddy Muddy 121 32.2 46.18.5 4 4,450 Muddy Lupine 121 31.8 46 18.7 3 4,398 Spring Cr Spring Cr. 121 33.5 46 20.4 unknown 3,900 Goal of the Management Plans Maintain and improve grassland/forb habitat at known occupancy meadow sites to ensure continued occupancy by mardon skipper butterfly as well as other important pollinator species such as western bumble bee. 1 Introduction On the Gifford Pinchot National Forest (GPNF), mardon skippers were first detected on the Mt. Adams Ranger District (MTA) in 2000 and on Cowlitz Valley Ranger District (CVRD) in 2002. Mardon skippers are known to inhabit ten, upland dry grassy meadows on the CVRD. Portions of the meadows are mesic and are unsuitable mardon skipper habitat. -

Butterflies and Moths of San Bernardino County, California
Heliothis ononis Flax Bollworm Moth Coptotriche aenea Blackberry Leafminer Argyresthia canadensis Apyrrothrix araxes Dull Firetip Phocides pigmalion Mangrove Skipper Phocides belus Belus Skipper Phocides palemon Guava Skipper Phocides urania Urania skipper Proteides mercurius Mercurial Skipper Epargyreus zestos Zestos Skipper Epargyreus clarus Silver-spotted Skipper Epargyreus spanna Hispaniolan Silverdrop Epargyreus exadeus Broken Silverdrop Polygonus leo Hammock Skipper Polygonus savigny Manuel's Skipper Chioides albofasciatus White-striped Longtail Chioides zilpa Zilpa Longtail Chioides ixion Hispaniolan Longtail Aguna asander Gold-spotted Aguna Aguna claxon Emerald Aguna Aguna metophis Tailed Aguna Typhedanus undulatus Mottled Longtail Typhedanus ampyx Gold-tufted Skipper Polythrix octomaculata Eight-spotted Longtail Polythrix mexicanus Mexican Longtail Polythrix asine Asine Longtail Polythrix caunus (Herrich-Schäffer, 1869) Zestusa dorus Short-tailed Skipper Codatractus carlos Carlos' Mottled-Skipper Codatractus alcaeus White-crescent Longtail Codatractus yucatanus Yucatan Mottled-Skipper Codatractus arizonensis Arizona Skipper Codatractus valeriana Valeriana Skipper Urbanus proteus Long-tailed Skipper Urbanus viterboana Bluish Longtail Urbanus belli Double-striped Longtail Urbanus pronus Pronus Longtail Urbanus esmeraldus Esmeralda Longtail Urbanus evona Turquoise Longtail Urbanus dorantes Dorantes Longtail Urbanus teleus Teleus Longtail Urbanus tanna Tanna Longtail Urbanus simplicius Plain Longtail Urbanus procne Brown Longtail -

Field Checklist of the Butterflies of Sonora, Mexico
Field Checklist Field Checklist of of the Butterfl ies of Sonora, Mexico The Butterfl ies of Sonora, Mexico List Compiled by Jim P. Brock Checklists available at Mexico Birding Website March 2009 http://MexicoBirding.com Kurt Radamaker Checklist Locality __________________________________ Observer(s) _______________________________ of the 1 Date __________Time ______ Total Species ____ Butterfl ies of Mexico Weather __________________________________ Remarks __________________________________ This checklist is a direct result of the work of Jim P. Brock's col- lecting and research in Sonora, Mexico since 1984. Locality __________________________________ Observer(s) _______________________________ 2 Date __________Time ______ Total Species ____ Weather __________________________________ Remarks __________________________________ Locality __________________________________ Observer(s) _______________________________ 3 Date __________Time ______ Total Species ____ Weather __________________________________ Remarks __________________________________ Locality __________________________________ Observer(s) _______________________________ 4 Date __________Time ______ Total Species ____ Weather __________________________________ Remarks __________________________________ Locality __________________________________ Observer(s) _______________________________ 5 Date __________Time ______ Total Species ____ Weather __________________________________ Booklet Design by Remarks __________________________________ Kurt and Cindy Radamaker March 2009 1 2 -

The Nectar Sources and Flower Preferences of the Poweshiek Skipperling (Oarisma Poweshiek) in Manitoba
The nectar sources and flower preferences of the Poweshiek Skipperling (Oarisma poweshiek) in Manitoba. Sarah Jericho Semmler Supervisor: Dr. A. R. Westwood A thesis submitted in partial fulfillment of the Honours Thesis (BIOL 4111/6) Course Department of Biology University of Winnipeg 2010 Abstract The Poweshiek Skipperling, Oarisma poweshiek, is a threatened butterfly found within the 2300 ha Tall Grass Prairie Preserve in southern Manitoba. Land management practices to maintain prairie habitats in a natural state, primarily burning and grazing, have been linked to a reduction in Poweshiek Skipperling habitat. Management activities in prairie habitat can change flowering plant composition, which may reduce the amount or type of nectar sources available for adult butterflies. In this study nectar plant diversity and adult Poweshiek Skipperling flower utilization between two sites with different burn histories was assessed. Preferred nectar flower sources of the Poweshiek Skipperling included Rudbeckia hirta and Solidago ptarmicoides, with skipperlings showing a strong preference for a 2002 burn site versus a 2008 burn site. Flowering plant diversity increased in the 2002 burn over the Poweshiek Skipperling flight period in comparison to the 2008 burn. The 2002 burn had shorter, less dense grass cover as well as a greater number of flowering stems of R. hirta and S. ptarmicoides. The level of competition by arthropods for the nectar or basking area on R. hirta was similar between the 2002 and 2008 burn sites. Flower nectar analysis indicated that sugar concentrations in R. hirta and S. ptarmicoides were relatively low compared to other flowering species during the flight period. The nectar tube length was similar in R. -

Download the Poweshiek Skipperling Status Assessment Update
STATUS ASSESSMENT UPDATE (2010) Poweshiek Skipperling Oarisma poweshiek (Parker) (Lepidoptera: Hesperiidae) Illinois, Indiana, Iowa, Michigan, Minnesota North Dakota, South Dakota, Wisconsin Contract #301818M448 By Gerald Selby Ecological and GIS Services 807 North W Street Indianola, IA 50125 For U.S. Fish and Wildlife Service Twin Cities Ecological Services Field Office 4101 E. 80th St. Bloomington, MN 55425 November 2010 Disclaimer This document is a compilation of biological data and a description of past, present, and likely future threats to Poweshiek skipperling (Oarisma poweshiek). It does not represent a decision by the U.S. Fish and Wildlife Service (Service) on whether this taxon should be designated as a candidate species for listing as threatened or endangered under the Federal Endangered Species Act. That decision will be made by the Service after reviewing this document; other relevant biological and threat data not included herein; and all relevant laws, regulations, and policies. The result of the decision will be posted on the Service's Region 3 Web site (refer to: http://www.fws.gov/midwest/endangered/lists/concern.html). If designated as a candidate species, the taxon will subsequently be added to the Service's candidate species list that is periodically published in the Federal Register and posted on the World Wide Web (refer to: http://endangered.fws.gov/wildlife.html). Even if the taxon does not warrant candidate status it should benefit from the conservation recommendations that are contained in this document. Suggested Citation: Selby, G. 2010. Status assessment update (2010): Poweshiek skipperling (Oarisma poweshiek (Parker)) (Lepidoptera: Hesperiidae). Prepared for Twin Cities Ecological Services Field Office, U.S. -

Minutes from the Poweshiek Skipperling Workshop
2011 Minutes from the Poweshiek Skipperling Workshop Photo: J. Dupont Winnipeg, Manitoba March 24th and 25th The minutes below represent the collective views captured and opinions of the experts who gathered to discuss the Poweshiek Skipperling. The views expressed in this document don’t represent the views of any one participant. The Workshop was led by the Nature Conservancy of Canada, Manitoba Region, and the minutes were summarized from audio recording and written notes by Jaimée Dupont. We would like to extend huge thank you to all of the individuals and organizations that attended and contributed to the workshop. We would also like to thank our sponsors: Abstract The objectives of this workshop were to bring together the Poweshiek Skipperling experts and those responsible for managing the species’ habitat from across its range, and open the lines of communication between them. Key goals of this workshop included identifying common trends amongst populations, discussing potential causes of these trends, identifying key research gaps and discussing how to fill these gaps. Workshop discussions provided immediate feedback and direction for National Recovery Planning and Implementation efforts that are currently underway in Canada and the USA. Conservation actions that should occur (range-wide and locally) were identified to ensure ongoing persistence of the species. The dramatic and seemingly concurrent declines seen locally were, unfortunately, echoed by participants from across the range (with the exception of Michigan). Workshop participants identified several potential causes of the species’ range- wide decline. Several potential factors that may be operating on a range-wide scale were discussed, and several lines of investigation were identified as critical research needs. -

Native Grasses Benefit Butterflies and Moths Diane M
AFNR HORTICULTURAL SCIENCE Native Grasses Benefit Butterflies and Moths Diane M. Narem and Mary H. Meyer more than three plant families (Bernays & NATIVE GRASSES AND LEPIDOPTERA Graham 1988). Native grasses are low maintenance, drought Studies in agricultural and urban landscapes tolerant plants that provide benefits to the have shown that patches with greater landscape, including minimizing soil erosion richness of native species had higher and increasing organic matter. Native grasses richness and abundance of butterflies (Ries also provide food and shelter for numerous et al. 2001; Collinge et al. 2003) and butterfly species of butterfly and moth larvae. These and moth larvae (Burghardt et al. 2008). caterpillars use the grasses in a variety of ways. Some species feed on them by boring into the stem, mining the inside of a leaf, or IMPORTANCE OF LEPIDOPTERA building a shelter using grass leaves and silk. Lepidoptera are an important part of the ecosystem: They are an important food source for rodents, bats, birds (particularly young birds), spiders and other insects They are pollinators of wild ecosystems. Terms: Lepidoptera - Order of insects that includes moths and butterflies Dakota skipper shelter in prairie dropseed plant literature review – a scholarly paper that IMPORTANT OF NATIVE PLANTS summarizes the current knowledge of a particular topic. Native plant species support more native graminoid – herbaceous plant with a grass-like Lepidoptera species as host and food plants morphology, includes grasses, sedges, and rushes than exotic plant species. This is partially due to the host-specificity of many species richness - the number of different species Lepidoptera that have evolved to feed on represented in an ecological community, certain species, genus, or families of plants. -

Final Lower Rio Grande Valley and Santa Ana National Wildlife
Final Lower Rio Grande Valley and Santa Ana National Wildlife Refuges Comprehensive Conservation Plan September 1997 (Reprint March 1999) U.S. Fish and Wildlife Service U.S. Department of the Interior Cover Artwork by Brian Cobble Table of Contents VISION........................................................................................................................................... 5 Executive Summary................................................................................................................... 6 1.0 Introduction and Regional Setting................................................................................. 8 1.1 LRGV Challenges............................................................................................... 8 2.0 Planning Perspectives and Considerations................................................................ 9 2.1 National Wildlife Refuge System ................................................................... 9 2.2 The Service & Ecosystem Management ...................................................... 9 2.3 Refuge Complex and Management Districts........................................... 10 2.4 Laguna Atascosa NWR -- A Partner with LRGV NWR............................ 10 2.5 Planning Perspectives.................................................................................... 10 2.6 The Issues.......................................................................................................... 11 2.7 The Need for Action........................................................................................ -

Oviposition Selection in Montane Habitats, Biological Conservation
Biological Conservation 143 (2010) 862–872 Contents lists available at ScienceDirect Biological Conservation journal homepage: www.elsevier.com/locate/biocon Oviposition selection by a rare grass skipper Polites mardon in montane habitats: Advancing ecological understanding to develop conservation strategies Loni J. Beyer, Cheryl B. Schultz * Washington State University Vancouver, 14204 NE Salmon Creek Ave, Vancouver, WA 98686, USA article info abstract Article history: The Grass skipper subfamily (Hesperiinae) includes many at risk species across the globe. Conservation Received 31 March 2009 efforts for these skippers are hindered by insufficient information about their basic biology. Mardon skip- Received in revised form 3 November 2009 per (Polites mardon) is declining throughout its range. We surveyed mardon oviposition across nine study Accepted 25 December 2009 meadows in the Gifford Pinchot National Forest of Washington State. We conducted habitat surveys with Available online 21 January 2010 respect to oviposition (n = 269) and random (n = 270) locations, recording data on over 50 variables. Mar- don oviposited on 23 different graminoid species, yet are selective for specific graminoids within mead- Keywords: ows. Most frequent ovipositions across meadows occurred on Festuca idahoensis and Poa pratensis Butterfly (accounting for 112 of 269 total oviposition observations). Discriminant Function Analyses revealed that Graminoids Habitat preference mardon habitat was too variable to detect oviposition selection across study meadows, yet there was 2 Host selection strong selection occurring within meadows (r ranging from 0.82 to 0.99). Variables important to within Life history meadow selection were graminoid cover, height, and community; oviposition plant structure (leaf den- Management sity, height, area); insolation factors (tree abundance and canopy shading); and litter layer factors (cover Meadow and depth). -

The Lepidopterists' News
The Lepidopterists' News THE MONTHLY NEWSLETTER OF THE LEPIDOPTERISTS' SOCIETY P. O. Box 104, Cambridge 38, Massachusetts • Edited by C. 1. REMINGTON and H. K. CLENCH Vol. ,I, No.7 November, 194'7 There has always been a tendency for Lepidopterists to segregate themselves from other entomologists, often with the result that they fail to apply advancement of other phases of entomology (and zoology) and lose the balanced approach necessary to good scientific work. A primary aim of the Lep.SQc.,through the !ffi1dS, ' is to reduce ,this barrier. In North America there are several local ,entomological societies , holding regular me-etings and in some cases publishing their own general journal, There is one correlating SOCiety, THE ENTOMOLOGICAL SOCIRTY OF AMERICA~ which ,has members from all parts 'of the continent. Lepid opterists should be aware of its existence, its functions, and its ac tivities. It is hoped that all Lep.Soc. members devoting much time to entomological research will want to become ' members of the E.S.A. if they have not yet done so. A short aceount of ,the history and activi ties of this Society will help to acquaint Lep_ Soc. members with it. T,RE 'ENTOl'/fOLOGICAL SOCIETY OF AMERICA was .organized in 1906 as a re sult of a spontaneous demand, and under the leadership of Prof. John H. Comstock, Dr. hilliam Morton \meeler, Dr. L.O. Howard,' Dr. Henry Skin ner, J. Chester Bradley, and others. By the end of its first year the Society had over 400 members and had Professor Comstock as its first president.