Apomixis in Neotropical Vegetation
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Eupatorieae: Asteraceae), Tratamento Taxonômico E Sinopse De Symphyopappus , E Anatomia Floral Do Clado Grazielia/Symphyopappus
ERIC KOITI OKIYAMA HATTORI FILOGENIA MOLECULAR DA SUBTRIBO DISYNAPHIINAE (EUPATORIEAE: ASTERACEAE), TRATAMENTO TAXONÔMICO E SINOPSE DE SYMPHYOPAPPUS , E ANATOMIA FLORAL DO CLADO GRAZIELIA/SYMPHYOPAPPUS Tese apresentada ao Programa de Pós-Graduação em Biologia Vegetal do Departamento de Botânica do Instituto de Ciências Biológicas da Universidade Federal de Minas Gerais, como requisito parcial à obtenção do título de Doutor em Biologia Vegetal. Área de Concentração: Taxonomia Vegetal BELO HORIZONTE – MG 2013 ii ERIC KOITI OKIYAMA HATTORI FILOGENIA MOLECULAR DA SUBTRIBO DISYNAPHIINAE (EUPATORIEAE: ASTERACEAE), TRATAMENTO TAXONÔMICO E SINOPSE DE SYMPHYOPAPPUS, E ANATOMIA FLORAL DO CLADO GRAZIELIA/SYMPHYOPAPPUS Tese apresentada ao Programa de Pós-Graduação em Biologia Vegetal do Departamento de Botânica do Instituto de Ciências Biológicas da Universidade Federal de Minas Gerais, como requisito parcial à obtenção do título de Doutor em Biologia Vegetal. Área de Concentração: Taxonomia Vegetal Orientador: Prof. Dr. João Aguiar Nogueira Batista Universidade Federal de Minas Gerais Coorientador: Profa. Dra. Denise Maria Trombert de Oliveira Universidade Federal de Minas Gerais Prof. Dr Jimi Naoki Nakajima Universidade Federal de Uberlândia BELO HORIZONTE – MG 2013 !"#$ %&''()*+$,)*-$.(*'*$/0*1&2&3$ $ $$$$$$4*5(678*&$2(57-95&)$:&$;9<')*<($:*;18&=>**8&7$?3$@3$.*86$A$%3$?(<3$ $ B,9=&'()*7&7C$D;'7)&-7&7E+$&8&'(2*&$F5()&5$:($-5&:($G)&H*75*&IJ12=>1(=&==9;$ 7$)7K*;L($'&M(8N2*-&$:7$J12=>1(=&==9;$O9)-H3$P2&89;-)*'(Q$I$,)*-$.(*'*$ /0*1&2&$%&''()*3$R$S!T#3$ -

New Species and Combinations of Apocynaceae, Bignoniaceae, Clethraceae, and Cunoniaceae from the Neotropics
Anales del Jardín Botánico de Madrid 75 (2): e071 https://doi.org/10.3989/ajbm.2499 ISSN: 0211-1322 [email protected], http://rjb.revistas.csic.es/index.php/rjb Copyright: © 2018 CSIC. This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial (by-nc) Spain 4.0 License. New species and combinations of Apocynaceae, Bignoniaceae, Clethraceae, and Cunoniaceae from the Neotropics Juan Francisco Morales 1,2,3 1 Missouri Botanical Garden 4344 Shaw Blvd. St. Louis, MO 63110, USA. 2 Bayreuth Center of Ecology and Environmental Research (BayCEER), University of Bayreuth, Universitätstrasse 30, 95447 Bayreuth, Germany. 3 Doctorado en Ciencias Naturales para el Desarrollo (DOCINADE), Universidad Estatal a Distancia, 474–2050 Montes de Oca, Costa Rica. [email protected], https://orcid.org/0000-0002-8906-8567 Abstract. Mandevilla arenicola J.F.Morales sp. nov. from Brazil, Clethra Resumen. Se describen e ilustran Mandevilla arenicola J.F.Morales secazu J.F.Morales sp. nov. from Costa Rica, and Weinmannia abstrusa sp. nov. de Brasil, Clethra secazu J.F.Morales sp. nov. de Costa Rica y J.F.Morales sp. nov. from Honduras are described and illustrated and Weinmannia abstrusa J.F.Morales sp. nov. de Honduras y se discuten their relationships with morphologically related species are discussed. sus relaciones con otras especies de morfología semejante. Se designan Lectotypes are designated for Anemopaegma tonduzianum Kraenzl., lectotipos para Anemopaegma tonduzianum Kraenzl., Bignonia Bignonia sarmentosa var. hirtella Benth. and Paragonia pyramidata var. sarmentosa var. hirtella Benth. and Paragonia pyramidata var. tomentosa tomentosa Bureau & K. Schum., as well as these last two names have Bureau & K.Schum., así como también se combinan estos dos últimos been combined. -

Otanewainuku ED (Report Prepared on 13 August 2013)
1 NZFRI collection wish list for Otanewainuku ED (Report prepared on 13 August 2013) Fern Ally Isolepis cernua Lycopodiaceae Isolepis inundata Lycopodium fastigiatum Isolepis marginata Lycopodium scariosum Isolepis pottsii Psilotaceae Isolepis prolifera Tmesipteris lanceolata Lepidosperma australe Lepidosperma laterale Gymnosperm Schoenoplectus pungens Cupressaceae Schoenoplectus tabernaemontani Chamaecyparis lawsoniana Schoenus apogon Cupressus macrocarpa Schoenus tendo Pinaceae Uncinia filiformis Pinus contorta Uncinia gracilenta Pinus patula Uncinia rupestris Pinus pinaster Uncinia scabra Pinus ponderosa Hemerocallidaceae Pinus radiata Dianella nigra Pinus strobus Phormium cookianum subsp. hookeri Podocarpaceae Phormium tenax Podocarpus totara var. totara Iridaceae Prumnopitys taxifolia Crocosmia xcrocosmiiflora Libertia grandiflora Monocotyledon Libertia ixioides Agapanthaceae Watsonia bulbillifera Agapanthus praecox Juncaceae Alliaceae Juncus articulatus Allium triquetrum Juncus australis Araceae Juncus conglomeratus Alocasia brisbanensis Juncus distegus Arum italicum Juncus edgariae Lemna minor Juncus effusus var. effusus Zantedeschia aethiopica Juncus sarophorus Arecaceae Juncus tenuis var. tenuis Rhopalostylis sapida Luzula congesta Asparagaceae Luzula multiflora Asparagus aethiopicus Luzula picta var. limosa Asparagus asparagoides Orchidaceae Cordyline australis x banksii Acianthus sinclairii Cordyline banksii x pumilio Aporostylis bifolia Asteliaceae Corunastylis nuda Collospermum microspermum Diplodium alobulum Commelinaceae -

Checklist Das Spermatophyta Do Estado De São Paulo, Brasil
Biota Neotrop., vol. 11(Supl.1) Checklist das Spermatophyta do Estado de São Paulo, Brasil Maria das Graças Lapa Wanderley1,10, George John Shepherd2, Suzana Ehlin Martins1, Tiago Egger Moellwald Duque Estrada3, Rebeca Politano Romanini1, Ingrid Koch4, José Rubens Pirani5, Therezinha Sant’Anna Melhem1, Ana Maria Giulietti Harley6, Luiza Sumiko Kinoshita2, Mara Angelina Galvão Magenta7, Hilda Maria Longhi Wagner8, Fábio de Barros9, Lúcia Garcez Lohmann5, Maria do Carmo Estanislau do Amaral2, Inês Cordeiro1, Sonia Aragaki1, Rosângela Simão Bianchini1 & Gerleni Lopes Esteves1 1Núcleo de Pesquisa Herbário do Estado, Instituto de Botânica, CP 68041, CEP 04045-972, São Paulo, SP, Brasil 2Departamento de Biologia Vegetal, Instituto de Biologia, Universidade Estadual de Campinas – UNICAMP, CP 6109, CEP 13083-970, Campinas, SP, Brasil 3Programa Biota/FAPESP, Departamento de Biologia Vegetal, Instituto de Biologia, Universidade Estadual de Campinas – UNICAMP, CP 6109, CEP 13083-970, Campinas, SP, Brasil 4Universidade Federal de São Carlos – UFSCar, Rod. João Leme dos Santos, Km 110, SP-264, Itinga, CEP 18052-780, Sorocaba, SP, Brasil 5Departamento de Botânica – IBUSP, Universidade de São Paulo – USP, Rua do Matão, 277, CEP 05508-090, Cidade Universitária, Butantã, São Paulo, SP, Brasil 6Departamento de Ciências Biológicas, Universidade Estadual de Feira de Santana – UEFS, Av. Transnordestina, s/n, Novo Horizonte, CEP 44036-900, Feira de Santana, BA, Brasil 7Universidade Santa Cecília – UNISANTA, R. Dr. Oswaldo Cruz, 266, Boqueirão, CEP 11045-907, -

Redalyc.ARE OUR ORCHIDS SAFE DOWN UNDER?
Lankesteriana International Journal on Orchidology ISSN: 1409-3871 [email protected] Universidad de Costa Rica Costa Rica BACKHOUSE, GARY N. ARE OUR ORCHIDS SAFE DOWN UNDER? A NATIONAL ASSESSMENT OF THREATENED ORCHIDS IN AUSTRALIA Lankesteriana International Journal on Orchidology, vol. 7, núm. 1-2, marzo, 2007, pp. 28- 43 Universidad de Costa Rica Cartago, Costa Rica Available in: http://www.redalyc.org/articulo.oa?id=44339813005 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative LANKESTERIANA 7(1-2): 28-43. 2007. ARE OUR ORCHIDS SAFE DOWN UNDER? A NATIONAL ASSESSMENT OF THREATENED ORCHIDS IN AUSTRALIA GARY N. BACKHOUSE Biodiversity and Ecosystem Services Division, Department of Sustainability and Environment 8 Nicholson Street, East Melbourne, Victoria 3002 Australia [email protected] KEY WORDS:threatened orchids Australia conservation status Introduction Many orchid species are included in this list. This paper examines the listing process for threatened Australia has about 1700 species of orchids, com- orchids in Australia, compares regional and national prising about 1300 named species in about 190 gen- lists of threatened orchids, and provides recommen- era, plus at least 400 undescribed species (Jones dations for improving the process of listing regionally 2006, pers. comm.). About 1400 species (82%) are and nationally threatened orchids. geophytes, almost all deciduous, seasonal species, while 300 species (18%) are evergreen epiphytes Methods and/or lithophytes. At least 95% of this orchid flora is endemic to Australia. -
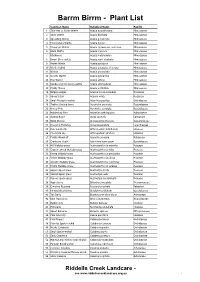
Botanical Name
Barrm Birrm - Plant List Common Name Botanical Name Family 1 Thin-leaf or Snake Wattle Acacia aculeatissima Mimosaceae 2 Silver Wattle Acacia dealbata Mimosaceae 3 Spreading Wattle Acacia genistifolia Mimosaceae 4 Ploughshare Wattle Acacia gunnii Mimosaceae 5 Cinnamon Wattle Acacia leprosa var. uninervia Mimosaceae 6 Black Wattle Acacia mearnsii Mimosaceae 7 Blackwood Acacia melanoxylon Mimosaceae 8 Dwarf Silver-wattle Acacia nano-dealbata Mimosaceae 9 Hedge Wattle Acacia paradoxa Mimosaceae 10 Wattle hybrid Acacia paradoxa x leprosa Mimosaceae 11 Wirilda Acacia provincialis Mimosaceae 12 Golden Wattle Acacia pycnantha Mimosaceae 13 Hop Wattle Acacia stricta Mimosaceae 14 Dandenong Cinnamon-wattle Acacia strictophylla Mimosaceae 15 Prickly Moses Acacia verticillata Mimosaceae 16 Bidgee-widgee Acaena novae-zelandiae Rosaceae 17 Sheep's Burr Acaena ovina Rosaceae 18 Small Mosquito-orchid Acianthus pusillus Orchidaceae 19 Trailing Ground-berry Acrotriche prostrata Epacridaceae 20 Honey Pots Acrotriche serrulata Epacridaceae 21 Maidenhair Fern Adiantum aethiopicum Adiantaceae 22 Austral Bugle Ajuga australis Lamiaceae 23 Black Sheoak Allocasuarina littoralis Casuarinaceae 24 Drooping Mistletoe Amyema pendula Loranthaceae 25 Pale Vanilla-lily Arthropodium milleflorum Liliaceae 26 Chocolate Lily Arthropodium strictum Liliaceae 27 Prickly Woodruff Asperula scoparia Rubiaceae 28 Cranberry Heath Astroloma humifusum Epacridaceae 29 Hill Wallaby-grass Austrodanthonia eriantha Poaceae 30 Copper-awned Wallaby-grass Austrodanthonia fulva Poaceae 31 -

Ethno-Knowledge of Medicinal Plants in a Community in the Eastern Amazon Etnoconhecimento De Plantas Medicinais Em Uma Comunidade Na Amazônia Oriental
Ethno-knowledge of medicinal plants in a community in the eastern Amazon Etnoconhecimento de plantas medicinais em uma comunidade na Amazônia oriental Luiz L. C. Moraes1, João L. Freitas2, João R. Matos Filho1, Raullyan B. L. Silva2, César H. A. Borges1 and Adriano C. Santos3,* 1 Universidade do Estado do Amapá-UEAP, Macapá, Amapá, Brazil 2 Instituto de Pesquisas Científicas e Tecnológicas do Estado do Amapá – IEPA, Macapá, Brazil 3 Instituto de Florestas do Estado do Amapá – IEF/AP, Macapá, Brazil (*E-mail: [email protected]) https://doi.org/10.19084/rca.15625 Received/recebido: 2018.11.19 Accepted/aceite: 2019.01.16 ABSTRACT The Amazon has many plant species with potential for medicinal use and a vast traditional knowledge accumulated over the years by the traditional peoples that inhabit the region. The objective of this work was to document traditional knowledge regarding the use of medicinal plants by São Tomé community residents that had its physical area affected by the reservoir of a hydroelectric plant in the region of the Araguari river valley, Amapá, Brazil. Data were collected from May to October 2014. Non-probabilistic sampling (n = 15) and participant observation were used to select local experts who understood about the use of medicinal plants. Socio-demographic and ethnobotanical data were collected during semi-structured interviews. There were 45 species distributed in 31 botanical families, with indicative of medic- inal use by local informants. The main parts used of the plants were bark and leaves. Keywords: rural communities, ethnospecies, hydroelectric, ethnobotany RESUMO A Amazônia possui muitas espécies vegetais com potencial para uso medicinal e um vasto conhecimento tradicional acumulado ao longo dos anos pelos povos tradicionais que habitam a região. -

ABSTRACTS 117 Systematics Section, BSA / ASPT / IOPB
Systematics Section, BSA / ASPT / IOPB 466 HARDY, CHRISTOPHER R.1,2*, JERROLD I DAVIS1, breeding system. This effectively reproductively isolates the species. ROBERT B. FADEN3, AND DENNIS W. STEVENSON1,2 Previous studies have provided extensive genetic, phylogenetic and 1Bailey Hortorium, Cornell University, Ithaca, NY 14853; 2New York natural selection data which allow for a rare opportunity to now Botanical Garden, Bronx, NY 10458; 3Dept. of Botany, National study and interpret ontogenetic changes as sources of evolutionary Museum of Natural History, Smithsonian Institution, Washington, novelties in floral form. Three populations of M. cardinalis and four DC 20560 populations of M. lewisii (representing both described races) were studied from initiation of floral apex to anthesis using SEM and light Phylogenetics of Cochliostema, Geogenanthus, and microscopy. Allometric analyses were conducted on data derived an undescribed genus (Commelinaceae) using from floral organs. Sympatric populations of the species from morphology and DNA sequence data from 26S, 5S- Yosemite National Park were compared. Calyces of M. lewisii initi- NTS, rbcL, and trnL-F loci ate later than those of M. cardinalis relative to the inner whorls, and sepals are taller and more acute. Relative times of initiation of phylogenetic study was conducted on a group of three small petals, sepals and pistil are similar in both species. Petal shapes dif- genera of neotropical Commelinaceae that exhibit a variety fer between species throughout development. Corolla aperture of unusual floral morphologies and habits. Morphological A shape becomes dorso-ventrally narrow during development of M. characters and DNA sequence data from plastid (rbcL, trnL-F) and lewisii, and laterally narrow in M. -

Unearthing Belowground Bud Banks in Fire-Prone Ecosystems
Unearthing belowground bud banks in fire-prone ecosystems 1 2 3 Author for correspondence: Juli G. Pausas , Byron B. Lamont , Susana Paula , Beatriz Appezzato-da- Juli G. Pausas 4 5 Glo'ria and Alessandra Fidelis Tel: +34 963 424124 1CIDE-CSIC, C. Naquera Km 4.5, Montcada, Valencia 46113, Spain; 2Department of Environment and Agriculture, Curtin Email [email protected] University, PO Box U1987, Perth, WA 6845, Australia; 3ICAEV, Universidad Austral de Chile, Campus Isla Teja, Casilla 567, Valdivia, Chile; 4Depto Ci^encias Biologicas,' Universidade de Sao Paulo, Av P'adua Dias 11., CEP 13418-900, Piracicaba, SP, Brazil; 5Instituto de Bioci^encias, Vegetation Ecology Lab, Universidade Estadual Paulista (UNESP), Av. 24-A 1515, 13506-900 Rio Claro, Brazil Summary To be published in New Phytologist (2018) Despite long-time awareness of the importance of the location of buds in plant biology, research doi: 10.1111/nph.14982 on belowground bud banks has been scant. Terms such as lignotuber, xylopodium and sobole, all referring to belowground bud-bearing structures, are used inconsistently in the literature. Key words: bud bank, fire-prone ecosystems, Because soil efficiently insulates meristems from the heat of fire, concealing buds below ground lignotuber, resprouting, rhizome, xylopodium. provides fitness benefits in fire-prone ecosystems. Thus, in these ecosystems, there is a remarkable diversity of bud-bearing structures. There are at least six locations where belowground buds are stored: roots, root crown, rhizomes, woody burls, fleshy -

Volume Ii Tomo Ii Diagnosis Biotic Environmen
Pöyry Tecnologia Ltda. Av. Alfredo Egídio de Souza Aranha, 100 Bloco B - 5° andar 04726-170 São Paulo - SP BRASIL Tel. +55 11 3472 6955 Fax +55 11 3472 6980 ENVIRONMENTAL IMPACT E-mail: [email protected] STUDY (EIA-RIMA) Date 19.10.2018 N° Reference 109000573-001-0000-E-1501 Page 1 LD Celulose S.A. Dissolving pulp mill in Indianópolis and Araguari, Minas Gerais VOLUME II – ENVIRONMENTAL DIAGNOSIS TOMO II – BIOTIC ENVIRONMENT Content Annex Distribution LD Celulose S.A. E PÖYRY - Orig. 19/10/18 –hbo 19/10/18 – bvv 19/10/18 – hfw 19/10/18 – hfw Para informação Rev. Data/Autor Data/Verificado Data/Aprovado Data/Autorizado Observações 109000573-001-0000-E-1501 2 SUMARY 8.3 Biotic Environment ................................................................................................................ 8 8.3.1 Objective .................................................................................................................... 8 8.3.2 Studied Area ............................................................................................................... 9 8.3.3 Regional Context ...................................................................................................... 10 8.3.4 Terrestrian Flora and Fauna....................................................................................... 15 8.3.5 Aquatic fauna .......................................................................................................... 167 8.3.6 Conservation Units (UC) and Priority Areas for Biodiversity Conservation (APCB) 219 8.3.7 -

Upper Montane Grassland Structure Within Six Subranges of Serra Do Mar, Southern Brazil1
Hoehnea 43(3): 401-435, 18 tab., 3 fig., 2016 http://dx.doi.org/10.1590/2236-8906-90/2015 Upper montane grassland structure within six subranges of Serra do Mar, Southern Brazil1 Maurício Bergamini Scheer2,4 and Alan Yukio Mocochinski3 Received: 11.01.2016; accepted: 8.07.2016 ABSTRACT - (Upper montane grassland structure within six subranges of Serra do Mar, Southern Brazil). The phytosociological structure of upper montane grasslands (high altitude grasslands) was studied in six subranges of Serra do Mar. Throughout 324 (1 m2) plot samples, we identified 195 taxa out of 280 taxa previously found in a floristic survey. Besides the general analysis of these communities, five physiognomies (synusiae) of these grasslands were previously determined based upon the species with greater cover. Cryptangium triquetrum and Croton mullerianus had the highest phytosociological importance value among the upper montane grasslands sampled in the present study. The first species was the most important of the grassy physiognomy of all sampled subranges and the second one of the shrubby physiognomy within three subranges. Chusquea pinifolia, Machaerina austrobrasiliensis, Deschampsia caespitosa, Gleichenella pectitata, Tibouchina dubia, Xyris stenophylla, Eryngium koehnearum and Eriochrysis holcoides were also structurally important. Although considerable species richness has been observed, the dominance of one or few species in the community was common in all subranges and physiognomies. In a brief comparison with upper montane vegetation studies (mainly on rocky outcrops) carried out in Southeastern Brazil, a low sharing of species was verified. Furthermore, the scarcity of studies in the literature regarding floristic and sociological structure of upper montane grasslands hampers a deeper analysis at level of species. -

Dimensions of Biodiversity
Dimensions of Biodiversity NATIONAL SCIENCE FOUNDATION CO-FUNDED BY 2010–2015 PROJECTS Introduction 4 Project Abstracts 2015 8 Project Updates 2014 30 Project Updates 2013 42 Project Updates 2012 56 Project Updates 2011 72 Project Updates 2010 88 FRONT COVER IMAGES A B f g h i k j C l m o n q p r D E IMAGE CREDIT THIS PAGE FRONT COVER a MBARI & d Steven Haddock f Steven Haddock k Steven Haddock o Carolyn Wessinger Peter Girguis e Carolyn g Erin Tripp l Lauren Schiebelhut p Steven Litaker b James Lendemer Wessinger h Marty Condon m Lawrence Smart q Sahand Pirbadian & c Matthew L. Lewis i Marty Condon n Verity Salmon Moh El-Naggar j Niklaus Grünwald r Marty Condon FIELD SITES Argentina France Singapore Australia French Guiana South Africa Bahamas French Polynesia Suriname Belize Germany Spain Bermuda Iceland Sweden Bolivia Japan Switzerland Brazil Madagascar Tahiti Canada Malaysia Taiwan China Mexico Thailand Colombia Norway Trinidad Costa Rica Palau United States Czech Republic Panama United Kingdom Dominican Peru Venezuela Republic Philippines Labrador Sea Ecuador Poland North Atlantic Finland Puerto Rico Ocean Russia North Pacific Ocean Saudi Arabia COLLABORATORS Argentina Finland Palau Australia France Panama Brazil Germany Peru Canada Guam Russia INTERNATIONAL PARTNERS Chile India South Africa China Brazil China Indonesia Sri Lanka (NSFC) (FAPESP) Colombia Japan Sweden Costa Rica Kenya United Denmark Malaysia Kingdom Ecuador Mexico ACKNOWLEDGMENTS Many NSF staff members, too numerous to We thank Mina Ta and Matthew Pepper for mention individually, assisted in the development their graphic design contribution to the abstract and implementation of the Dimensions of booklet.