E Edulis ATION FLORISTIC ELEMENTS AS BASIS FOR
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thomas Lewinsohn with Paulo Inácio Prado USP Mário Almeida Neto UFG Adriana Almeida UFRN Leonardo Ré Jorge Unicamp ______Laboratório Interações Insetos-Plantas Depto
Phytophagous insects on flower heads of Neotropical Compositae Thomas Lewinsohn with Paulo Inácio Prado USP Mário Almeida Neto UFG Adriana Almeida UFRN Leonardo Ré Jorge Unicamp _________________________ Laboratório Interações Insetos-Plantas Depto. Biologia Animal, Inst. Biologia Unicamp – University of Campinas herbivores+plants: the multicellular majority Terry Erwin, 1982: “... as many as 30 million insects” Terry Erwin who’s who among the herbivorous insects beetles moths, butterflies flies, midges sawflies bugs, aphids grasshoppers thrips walking sticks data sources: taxonomic studies taxonomy based on adults - what do larvae do? no host records unreliable host identification data sources: biocontrol surveys Carduus nutans with Rhynocyllus conicus (Curculionidae) data sources: community diversity studies • Plant samples (plots, individual trees) and • insect mass samples (net sweep, suction samples, fogging, light traps) Murdoch, Evans & Peterson 1972 adult insects on plants: herbivores or tourists? Insects and Compositae as ecological study systems A model system for herbivore evolution Solidago – Eurosta – parasitoids/predators A model system for population dynamics ragwort, Senecio jacobaea - cinnabar moth, Tyria jacobaeae Longitarsus Chromatomyia Melanagromyza metacommunity dynamics a field experiment From biocontrol surveys to ecological insights Biocontrol prospecting in South America Baccharis Daniel Gandolfo Gutierrezia Chromolaena odorata > Campuloclinium macrocephalum basic study design a suitable plant-herbivore system -

Eupatorieae: Asteraceae), Tratamento Taxonômico E Sinopse De Symphyopappus , E Anatomia Floral Do Clado Grazielia/Symphyopappus
ERIC KOITI OKIYAMA HATTORI FILOGENIA MOLECULAR DA SUBTRIBO DISYNAPHIINAE (EUPATORIEAE: ASTERACEAE), TRATAMENTO TAXONÔMICO E SINOPSE DE SYMPHYOPAPPUS , E ANATOMIA FLORAL DO CLADO GRAZIELIA/SYMPHYOPAPPUS Tese apresentada ao Programa de Pós-Graduação em Biologia Vegetal do Departamento de Botânica do Instituto de Ciências Biológicas da Universidade Federal de Minas Gerais, como requisito parcial à obtenção do título de Doutor em Biologia Vegetal. Área de Concentração: Taxonomia Vegetal BELO HORIZONTE – MG 2013 ii ERIC KOITI OKIYAMA HATTORI FILOGENIA MOLECULAR DA SUBTRIBO DISYNAPHIINAE (EUPATORIEAE: ASTERACEAE), TRATAMENTO TAXONÔMICO E SINOPSE DE SYMPHYOPAPPUS, E ANATOMIA FLORAL DO CLADO GRAZIELIA/SYMPHYOPAPPUS Tese apresentada ao Programa de Pós-Graduação em Biologia Vegetal do Departamento de Botânica do Instituto de Ciências Biológicas da Universidade Federal de Minas Gerais, como requisito parcial à obtenção do título de Doutor em Biologia Vegetal. Área de Concentração: Taxonomia Vegetal Orientador: Prof. Dr. João Aguiar Nogueira Batista Universidade Federal de Minas Gerais Coorientador: Profa. Dra. Denise Maria Trombert de Oliveira Universidade Federal de Minas Gerais Prof. Dr Jimi Naoki Nakajima Universidade Federal de Uberlândia BELO HORIZONTE – MG 2013 !"#$ %&''()*+$,)*-$.(*'*$/0*1&2&3$ $ $$$$$$4*5(678*&$2(57-95&)$:&$;9<')*<($:*;18&=>**8&7$?3$@3$.*86$A$%3$?(<3$ $ B,9=&'()*7&7C$D;'7)&-7&7E+$&8&'(2*&$F5()&5$:($-5&:($G)&H*75*&IJ12=>1(=&==9;$ 7$)7K*;L($'&M(8N2*-&$:7$J12=>1(=&==9;$O9)-H3$P2&89;-)*'(Q$I$,)*-$.(*'*$ /0*1&2&$%&''()*3$R$S!T#3$ -

Nutritional Disorders of Macronutrients in Bletia Catenulata
HORTSCIENCE 54(10):1836–1839. 2019. https://doi.org/10.21273/HORTSCI14284-19 Thus, the deficiency symptoms of a partic- ular nutrient are typical and may appear in several plant organs, such as leaves, stems, Nutritional Disorders of roots, and fruit. These symptoms assist in the nutritional evaluation of plants (Gontijo et al., Macronutrients in Bletia catenulata 2007). However, mineral deficiency symptoms present particular inter- and intraspecies re- Carlos Henrique Oliveira de David sponses as a result of gene expression and envi- Universidade Federal de Mato Grosso do Sul, Chapadao~ do Sul, Brazil ronmental factors (Hawkesford et al., 2012). The knowledge of the symptomatology Vespasiano Borges de Paiva Neto caused by the deficiency of a specific nutrient Universidade Federal do Vale do Sao~ Francisco, Petrolina, Brazil is fundamental to the use of this method of plant nutritional evaluation. Thus, the culti- Cid Naudi Silva Campos, Priscilla Maria da Silva Liber Lopes, vation of plants in protected systems using and Paulo Eduardo Teodoro culture medium is an essential tool for plant Universidade Federal de Mato Grosso do Sul, Chapadao~ do Sul, Brazil nutrition studies, especially those that induce nutritional deficiency (Prado, 2008). Renato de Mello Prado Studies involving nutritional disorders in Universidade Federal de Mato Grosso do Sul, Chapadao~ do Sul, Brazil; orchids are still incipient in the literature, Department of Soils and Fertilizers, Universidade Estadual Paulista ‘‘Julio specifically for B. catenulata. Therefore, nutri- de Mesquita Filho’’ (UNESP), Jaboticabal, Brazil tional management of this species has not yet been consolidated, which can affect plant yield Additional index words. Orchidaceae, nutritional disorder, plant nutrition and quality. -

Análisis Aeropalinológico Del Parque Nacional El Palmar
Bol. Soc. Argent. Bot. 52 (3) 2017 N. E. Muñoz et al. - Análisis aeropalinológico del Parque NacionalISSN El0373-580 Palmar X Bol. Soc. Argent. Bot. 52 (3): 473-496. 2017 ANÁLISIS AEROPALINOLÓGICO EN TRES ÁREAS DE VEGETACIÓN DENTRO DEL PARQUE NACIONAL EL PALMAR (COLÓN, ENTRE RÍOS) Y SU RELACIÓN CON LA VEGETACIÓN LOCAL Y REGIONAL NADIA E. MUÑOZ1, MERCEDES DI PASQUO1, FERNANDO BIGANZOLI2 y WILLIAM B. BATISTA2,3 Summary: Aeropalinological analysis in three vegetation areas within El Palmar National Park (Colón, Entre Ríos) and its relationship with the local and regional vegetation. The diversity of pollen rain monthly collected during two years (2011-2013) from the atmosphere in Tauber traps located at three sites in El Palmar National Park (Entre Ríos Province) is used to characterize the source vegetation. Site 1 is a mixed area composed of grassland, palm savanna, and wetland communities, site 2 is a grassland area and site 3 is a dense palm savanna. A total of 71 pollen-grain types grouped in 43 families coming from local, regional and extra- regional areas are identified. Of them, sixteen pollen types with more than 1% of Annual Pollen Influx in at least two samples were used in this analysis. Different factors involved in quali-quantitave changes of taxa during the observation interval (e.g. pollination affinity, origin of pollen grains, canopy effect, meteorological variables) are further considered. The floral composition of each site compared to their palynoassemblages revealed that site 2 is characterized by a high abundance of Asteraeceae-Asteroideae, with an increase in the value of Vernonia (Asteraceae Cichoroidea) and Lamiaceae during the second year. -

The Aquatic Macrophyte Flora of the Pandeiros River Wildlife Sanctuary
Check List 9(2): 415–424, 2013 © 2013 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution The Aquatic Macrophyte Flora of the Pandeiros River PECIES S Wildlife Sanctuary, Minas Gerais, Brazil OF Marco Otávio Dias Pivari 1*, Pedro Lage Viana 1 and Felipe Sá Fortes Leite 2 ISTS L 1 Universidade Federal de Minas Gerais, Instituto de Ciências Biológicas, Laboratório de Sistemática Vegetal. Avenida Antônio Carlos 6621. CEP 31270-010. Belo Horizonte, MG, Brazil. 2 Universidade Federal de Minas Gerais, Departamento de Zoologia. Avenida Antônio Carlos 6621. CEP 31270-010. Belo Horizonte, MG, Brazil. * Corresponding author. E-mail: [email protected] Abstract: The São Francisco River forms one of the main Brazilian hydrographic basins of ca. 645,000 km2. The Pandeiros River is a tributary situated on the left margin of the São Francisco and is considered a strategic component for conservation Sanctuary was carried out, using collections of botanical samples and examination of specimens at the BHCB Herbarium. of biodiversity of that hydrographic basin. An inventory of the aquatic macrophyte flora of the Pandeiros River Wildlife swamps. A total of 101 species was inventoried, distributed in 37 families (1 charophytes, 1 liverworts, 3 ferns and 32 Aquatic environments in the study area were classified as follows: the Pandeiros riverbed, floodplains, oxbow lakes, and representative. The area shows a high diversity in its aquatic macrophytes and has an important role in the conservation of biodiversityangiosperms) of and the 71region. genera. The species were classified into seven life forms, with the amphibian and rafted plants the more Introduction forests, and several aquatic macrophyte environments, Aquatic environments correspond to approximately associated with watercourses (Barbosa and Maillard 11% of the continental area of tropical regions (Rebouças 2010). -

Phylogenetic Placement of the Enigmatic Orchid Genera Thaia and Tangtsinia: Evidence from Molecular and Morphological Characters
TAXON 61 (1) • February 2012: 45–54 Xiang & al. • Phylogenetic placement of Thaia and Tangtsinia Phylogenetic placement of the enigmatic orchid genera Thaia and Tangtsinia: Evidence from molecular and morphological characters Xiao-Guo Xiang,1 De-Zhu Li,2 Wei-Tao Jin,1 Hai-Lang Zhou,1 Jian-Wu Li3 & Xiao-Hua Jin1 1 Herbarium & State Key Laboratory of Systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, P.R. China 2 Key Laboratory of Biodiversity and Biogeography, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan 650204, P.R. China 3 Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Menglun Township, Mengla County, Yunnan province 666303, P.R. China Author for correspondence: Xiao-Hua Jin, [email protected] Abstract The phylogenetic position of two enigmatic Asian orchid genera, Thaia and Tangtsinia, were inferred from molecular data and morphological evidence. An analysis of combined plastid data (rbcL + matK + psaB) using Bayesian and parsimony methods revealed that Thaia is a sister group to the higher epidendroids, and tribe Neottieae is polyphyletic unless Thaia is removed. Morphological evidence, such as plicate leaves and corms, the structure of the gynostemium and the micromorphol- ogy of pollinia, also indicates that Thaia should be excluded from Neottieae. Thaieae, a new tribe, is therefore tentatively established. Using Bayesian and parsimony methods, analyses of combined plastid and nuclear datasets (rbcL, matK, psaB, trnL-F, ITS, Xdh) confirmed that the monotypic genus Tangtsinia was nested within and is synonymous with the genus Cepha- lanthera, in which an apical stigma has evolved independently at least twice. -

Low Risk Aquarium and Pond Plants
Plant Identification Guide Low-risk aquarium and pond plants Planting these in your pond or aquarium is environmentally-friendly. Glossostigma elatinoides, image © Sonia Frimmel. One of the biggest threats to New Zealand’s waterbodies is the establishment and proliferation of weeds. The majority of New Zealand’s current aquatic weeds started out as aquarium and pond plants. To reduce the occurrence of new weeds becoming established in waterbodies this guide has been prepared to encourage the use of aquarium and pond plants that pose minimal risk to waterbodies. Guide prepared by Dr John Clayton, Paula Reeves, Paul Champion and Tracey Edwards, National Centre of Aquatic Biodiversity and Biosecurity, NIWA with funding from the Department of Conservation. The guides will be updated on a regular basis and will be available on the NIWA website: www.niwa.co.nz/ncabb/tools. Key to plant life-forms Sprawling marginal plants. Grow across the ground and out over water. Pond plants Short turf-like plants. Grow in shallow water on the edges of ponds and foreground of aquariums. Includes very small plants (up to 2-3 cm in height). Most species can grow both submerged (usually more erect) and emergent. Pond and aquarium plants Tall emergent plants. Can grow in water depths up to 2 m deep depending on the species. Usually tall reed-like plants but sometimes with broad leaves. Ideal for deeper ponds. Pond plants Free floating plants. These plants grow on the water surface and are not anchored to banks or bottom substrates. Pond and aquarium plants Floating-leaved plants. Water lily-type plants. -

C6 Noncarice Sedge
CYPERACEAE etal Got Sedge? Part Two revised 24 May 2015. Draft from Designs On Nature; Up Your C 25 SEDGES, FOINS COUPANTS, LAÎCHES, ROUCHES, ROUCHETTES, & some mostly wet things in the sedge family. Because Bill Gates has been shown to eat footnotes (burp!, & enjoy it), footnotes are (italicized in the body of the text) for their protection. Someone who can spell caespitose only won way has know imagination. Much of the following is taken verbatim from other works, & often not credited. There is often not a way to paraphrase or rewrite habitat or descriptive information without changing the meaning. I am responsible for any mistakes in quoting or otherwise. This is a learning tool, & a continuation of an idea of my friend & former employer, Jock Ingels, LaFayette Home Nursery, who hoped to present more available information about a plant in one easily accessible place, instead of scattered though numerous sources. This is a work in perpetual progress, a personal learning tool, full uv misstakes, & written as a personal means instead of a public end. Redundant, repetitive, superfluous, & contradictory information is present. It is being consolidated. CYPERACEAE Sauergrasgewächse SEDGES, aka BIESIES, SEGGEN Formally described in 1789 by De Jussieu. The family name is derived from the genus name Cyperus, from the Greek kupeiros, meaning sedge. Many species are grass-like, being tufted, with long, thin, narrow leaves, jointed stems, & branched inflorescence of small flowers, & are horticulturally lumped with grasses as graminoids. Archer (2005) suggests the term graminoid be used for true grasses, & cyperoid be used for sedges. (If physical anthropologists have hominoids & hominids, why don’t we have graminoids & graminids?) There are approximately 104 genera, 4 subfamilies, 14 tribes, & about 5000 species worldwide, with 27 genera & 843 species in North America (Ball et al 2002). -

Universidad Nacional De San Antonio Abad Del Cusco Facultad De Ciencias Escuela Profesional De Biología
Universidad Nacional de San Antonio Abad del Cusco Facultad de Ciencias Escuela Profesional de Biología MICROPROPAGACION DE ORQUÍDEAS A PARTIR DE SEMILLAS EN CONDICIONES DE CULTIVO IN VITRO Tesis Presentada por: Bachiller. Karina Flores Huisa Para Optar al Título Profesional de Biólogo Asesor: M. Sc. Máximo Américo Chacón Campana Cusco – Perú 2019 Dedicatoria 9 A Dios por protegerme cada día de mi vida. 9 A mis padres; Apolinaria y Francisco por darme la vida y ser un ejemplo a seguir. 9 A mis hermanos José Luis, Beatriz, Daniel, Juan Carlos, Milagros por sus palabras de aliento a seguir e inolvidables momentos compartidos en familia. 9 A mi mentor Dr. Luis Fernando Miranda Ángeles (ƚ) y familia por haber contribuido a mi formación profesional y todos sus consejos dados a lo largo de mi vida. Agradecimientos 9 A la Universidad Nacional de San Antonio Abad del Cusco por haberme formado en la Carrera de Ciencias Biológicas. 9 A la Universidad Nacional Agraria la Molina y al Instituto de Biotecnología (IBT) por haberme permitido continuar con mi formación profesional. 9 Un agradecimiento a mi asesor M.Sc. Blgo Máximo Américo Chacón Campana por su asesoramiento en la presente Tesis. 9 Al cuerpo docente de la Facultad de Ciencias, Escuela Profesional de Biología, por haber contribuido en mi formación profesional. 9 Mis agradecimientos a la Dra María E. Holgado Rojas, M.Sc Blga Gloria Calatayud Hermosa, al Blgo. Marcial Villafuerte Arriaga, por su ayuda en la colecta de orquídeas y sus valiosos aportes. 9 Al Blgo. Marco León Martínez por su donación de la especie de Epidendrum macrocarpum para la realización del trabajo. -
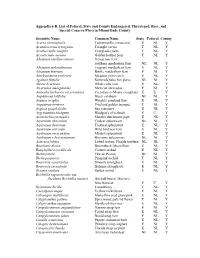
Conservation Appendix 6-B Listed Flora
Appendix 6-B. List of Federal, State and County Endangered, Threatened, Rare, and Special Concern Flora in Miami-Dade County Scientific Name Common Name State Federal County Acacia choriophylla Tamarindillo; cinnecord E NL Y Acanthocereus tetragenus Triangle cactus T NL Y Acoelorraphe wrightii Everglades palm T NL Y Acrostichum aureum Golden leather fern T NL Y Adiantum capillus-veneris Venus hair fern; southern maidenhair fern NL NL Y Adiantum melanoleucum Fragrant maidenhair fern E NL Y Adiantum tenerum Brittle maidenhair fern E NL Y Aeschynomene pratensis Meadow joint-vetch E NL Y Agalinis filifolia Seminole false fox glove NL NL Y Aletris bracteata White colic root E NL Y Alvaradoa amorphoides Mexican alvaradoa E NL Y Amorpha herbacea var.crenulata Crenulate (=Miami) leadplant E E Y Amphitecna latifolia Black calabash NL NL Y Anemia wrightii Wright's pineland fern E NL Y Angadenia berteroi Pineland golden trumpet T NL Y Argusia gnaphalodes Sea rosemary E NL Y Argythamnia blodgettii Blodgett's silverbush E C Y Aristolochia pentandra Marsh's dutchmans pipe E NL Y Asplenium abscissum Cutleaf spleenwort NL NL Y Asplenium dentatum Toothed spleenwort E NL Y Asplenium serratum Wild bird nest fern E NL Y Asplenium verecundum Modest spleenwort E NL Y Asplenium x biscaynianum Biscayne spleenwort NL NL Y Asteraea lobata Lobed croton; Florida treefern NL NL Y Baccharis dioica Broombush falsewillow E NL Y Basiphyllaea corallicola Carter's orchid E NL Y Bletia patula Flor de Pesmo NL NL Y Bletia purpurea Pinepink orchid T NL Y Bourreria cassinifolia Smooth strongback E NL Y Bourreria succulenta Bahama strongback E NL Y Brassia caudata Spider orchid E NL Y Brickellia eupatorioides var. -

Redalyc.EFFORTS to CONSERVE ENDANGERED TERRESTRIAL
Lankesteriana International Journal on Orchidology ISSN: 1409-3871 [email protected] Universidad de Costa Rica Costa Rica RANGEL-VILLAFRANCO, MONICA; ORTEGA-LARROCEA, M. PILAR EFFORTS TO CONSERVE ENDANGERED TERRESTRIAL ORCHIDS IN SITU AND EX SITU AT TWO NATURAL RESERVES WITHIN CENTRAL MEXICO Lankesteriana International Journal on Orchidology, vol. 7, núm. 1-2, marzo, 2007, pp. 326-333 Universidad de Costa Rica Cartago, Costa Rica Available in: http://www.redalyc.org/articulo.oa?id=44339813068 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative LANKESTERIANA 7(1-2): 326-333. 2007. EFFORTS TO CONSERVE ENDANGERED TERRESTRIAL ORCHIDS IN SITU AND EX SITU AT TWO NATURAL RESERVES WITHIN CENTRAL MEXICO 1 1,2 MONICA RANGEL-VILLAFRANCO & M. PILAR ORTEGA-LARROCEA 1 Departamento de Edafología, Instituto de Geología, Universidad Nacional Autónoma de México. Circuito Exterior de Ciudad Universitaria, México Distrito Federal, 04510. México. 2 Author for correspondence: [email protected] KEY WORDS: in situ conservation, ex situ conservation, orchid fungi isolation, seed banks The natural vegetation in and around Mexico City macrobulon, Epidendrum anisatu, Habenaria strictis- once harbored an unusually high number of plant and sima, Liparis greenwoodiana) (Hágsater et al. 2005). animal (insect) species, including endemics (Vázquez In contrast, in the Chichinautzin Area, eight types 1973, Ceballos & Galindo 1984, Rzedowski 1991). of vegetation can be found. An altitudinal gradient The high diversity in this region has been attributed joint with successive periods of volcanic activity to the unusual topography resulting from a series of are combined and pedogenetic processes through volcanic eruptions that ended ca. -

Checklist Das Spermatophyta Do Estado De São Paulo, Brasil
Biota Neotrop., vol. 11(Supl.1) Checklist das Spermatophyta do Estado de São Paulo, Brasil Maria das Graças Lapa Wanderley1,10, George John Shepherd2, Suzana Ehlin Martins1, Tiago Egger Moellwald Duque Estrada3, Rebeca Politano Romanini1, Ingrid Koch4, José Rubens Pirani5, Therezinha Sant’Anna Melhem1, Ana Maria Giulietti Harley6, Luiza Sumiko Kinoshita2, Mara Angelina Galvão Magenta7, Hilda Maria Longhi Wagner8, Fábio de Barros9, Lúcia Garcez Lohmann5, Maria do Carmo Estanislau do Amaral2, Inês Cordeiro1, Sonia Aragaki1, Rosângela Simão Bianchini1 & Gerleni Lopes Esteves1 1Núcleo de Pesquisa Herbário do Estado, Instituto de Botânica, CP 68041, CEP 04045-972, São Paulo, SP, Brasil 2Departamento de Biologia Vegetal, Instituto de Biologia, Universidade Estadual de Campinas – UNICAMP, CP 6109, CEP 13083-970, Campinas, SP, Brasil 3Programa Biota/FAPESP, Departamento de Biologia Vegetal, Instituto de Biologia, Universidade Estadual de Campinas – UNICAMP, CP 6109, CEP 13083-970, Campinas, SP, Brasil 4Universidade Federal de São Carlos – UFSCar, Rod. João Leme dos Santos, Km 110, SP-264, Itinga, CEP 18052-780, Sorocaba, SP, Brasil 5Departamento de Botânica – IBUSP, Universidade de São Paulo – USP, Rua do Matão, 277, CEP 05508-090, Cidade Universitária, Butantã, São Paulo, SP, Brasil 6Departamento de Ciências Biológicas, Universidade Estadual de Feira de Santana – UEFS, Av. Transnordestina, s/n, Novo Horizonte, CEP 44036-900, Feira de Santana, BA, Brasil 7Universidade Santa Cecília – UNISANTA, R. Dr. Oswaldo Cruz, 266, Boqueirão, CEP 11045-907,