Phylogeny, Character Evolution and the Systematics of Psilochilus (Triphoreae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Native Orchids in Southeast Alaska
Native Orchids in Southeast Alaska Marlin Bowles & Bob Armstrong 2019 Preface Southeast Alaska's rainforests, peatlands and alpine habitats support a wide variety of plant life. The composition of this vegetation is strongly influenced by patterns of plant distribution and geographical factors. For example, the ranges of some Asian plant species extend into Southeast Alaska by way of the Aleutian Islands; other species extend northward into this region along the Pacific coast or southward from central Alaska. Included in Southeast Alaska's vegetation are at least 27 native orchid species and varieties whose collective ranges extend from Mexico north to beyond the Arctic Circle, and from North America to northern Europe and Asia. These orchids survive in a delicate ecological balance, requiring specific insect pollinators for seed production, and mycorrhizal fungi that provide nutrients essential for seedling growth and survival of adult plants. These complex relationships can lead to vulnerability to human impacts. Orchids also tend to transplant poorly and typically perish without their fungal partners. They are best left to survive as important components of biodiversity as well as resources for our enjoyment. Our goal is to provide a useful description of Southeast Alaska's native orchids for readers who share enthusiasm for the natural environment and desire to learn more about our native orchids. This book addresses each of the native orchids found in the area of Southeast Alaska extending from Yakutat and the Yukon border south to Ketchikan and the British Columbia border. For each species, we include a brief description of its distribution, habitat, size, mode of reproduction, and pollination biology. -

November December 2010 Vol 67.3 Victoria Natural
NOVEMBER DECEMBER 2010 VOL 67.3 VICTORIA NATURAL HISTORY SOCIETY The Victoria Naturalist Vol. 67.3 (2010) 1 Published six times a year by the SUBMISSIONS VICTORIA NATURAL HISTORY SOCIETY, P.O. Box 5220, Station B, Victoria, BC V8R 6N4 Deadline for next issue: December 1, 2010 Contents © 2010 as credited. Send to: Claudia Copley ISSN 0049—612X Printed in Canada 657 Beaver Lake Road, Victoria BC V8Z 5N9 Editors: Claudia Copley, 250-479-6622, Penelope Edwards Phone: 250-479-6622 Desktop Publishing: Frances Hunter, 250-479-1956 e-mail: [email protected] Distribution: Tom Gillespie, Phyllis Henderson, Morwyn Marshall Printing: Fotoprint, 250-382-8218 Guidelines for Submissions Opinions expressed by contributors to The Victoria Naturalist Members are encouraged to submit articles, field trip reports, natural are not necessarily those of the Society. history notes, and book reviews with photographs or illustrations if possible. Photographs of natural history are appreciated along with VICTORIA NATURAL HISTORY SOCIETY documentation of location, species names and a date. Please label your Honorary Life Members Dr. Bill Austin, Mrs. Lyndis Davis, submission with your name, address, and phone number and provide a Mr. Tony Embleton, Mr. Tom Gillespie, Mrs. Peggy Goodwill, title. We request submission of typed, double-spaced copy in an IBM compatible word processing file on diskette, or by e-mail. Photos and Mr. David Stirling, Mr. Bruce Whittington slides, and diskettes submitted will be returned if a stamped, self- Officers: 2009-2010 addressed envelope is included with the material. Digital images are PRESIDENT: Darren Copley, 250-479-6622, [email protected] welcome, but they need to be high resolution: a minimum of 1200 x VICE-PRESIDENT: James Miskelly, 250-477-0490, [email protected] 1550 pixels, or 300 dpi at the size of photos in the magazine. -

Likely to Have Habitat Within Iras That ALLOW Road
Item 3a - Sensitive Species National Master List By Region and Species Group Not likely to have habitat within IRAs Not likely to have Federal Likely to have habitat that DO NOT ALLOW habitat within IRAs Candidate within IRAs that DO Likely to have habitat road (re)construction that ALLOW road Forest Service Species Under NOT ALLOW road within IRAs that ALLOW but could be (re)construction but Species Scientific Name Common Name Species Group Region ESA (re)construction? road (re)construction? affected? could be affected? Bufo boreas boreas Boreal Western Toad Amphibian 1 No Yes Yes No No Plethodon vandykei idahoensis Coeur D'Alene Salamander Amphibian 1 No Yes Yes No No Rana pipiens Northern Leopard Frog Amphibian 1 No Yes Yes No No Accipiter gentilis Northern Goshawk Bird 1 No Yes Yes No No Ammodramus bairdii Baird's Sparrow Bird 1 No No Yes No No Anthus spragueii Sprague's Pipit Bird 1 No No Yes No No Centrocercus urophasianus Sage Grouse Bird 1 No Yes Yes No No Cygnus buccinator Trumpeter Swan Bird 1 No Yes Yes No No Falco peregrinus anatum American Peregrine Falcon Bird 1 No Yes Yes No No Gavia immer Common Loon Bird 1 No Yes Yes No No Histrionicus histrionicus Harlequin Duck Bird 1 No Yes Yes No No Lanius ludovicianus Loggerhead Shrike Bird 1 No Yes Yes No No Oreortyx pictus Mountain Quail Bird 1 No Yes Yes No No Otus flammeolus Flammulated Owl Bird 1 No Yes Yes No No Picoides albolarvatus White-Headed Woodpecker Bird 1 No Yes Yes No No Picoides arcticus Black-Backed Woodpecker Bird 1 No Yes Yes No No Speotyto cunicularia Burrowing -

Guide to the Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- LILIACEAE
Guide to the Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- LILIACEAE LILIACEAE de Jussieu 1789 (Lily Family) (also see AGAVACEAE, ALLIACEAE, ALSTROEMERIACEAE, AMARYLLIDACEAE, ASPARAGACEAE, COLCHICACEAE, HEMEROCALLIDACEAE, HOSTACEAE, HYACINTHACEAE, HYPOXIDACEAE, MELANTHIACEAE, NARTHECIACEAE, RUSCACEAE, SMILACACEAE, THEMIDACEAE, TOFIELDIACEAE) As here interpreted narrowly, the Liliaceae constitutes about 11 genera and 550 species, of the Northern Hemisphere. There has been much recent investigation and re-interpretation of evidence regarding the upper-level taxonomy of the Liliales, with strong suggestions that the broad Liliaceae recognized by Cronquist (1981) is artificial and polyphyletic. Cronquist (1993) himself concurs, at least to a degree: "we still await a comprehensive reorganization of the lilies into several families more comparable to other recognized families of angiosperms." Dahlgren & Clifford (1982) and Dahlgren, Clifford, & Yeo (1985) synthesized an early phase in the modern revolution of monocot taxonomy. Since then, additional research, especially molecular (Duvall et al. 1993, Chase et al. 1993, Bogler & Simpson 1995, and many others), has strongly validated the general lines (and many details) of Dahlgren's arrangement. The most recent synthesis (Kubitzki 1998a) is followed as the basis for familial and generic taxonomy of the lilies and their relatives (see summary below). References: Angiosperm Phylogeny Group (1998, 2003); Tamura in Kubitzki (1998a). Our “liliaceous” genera (members of orders placed in the Lilianae) are therefore divided as shown below, largely following Kubitzki (1998a) and some more recent molecular analyses. ALISMATALES TOFIELDIACEAE: Pleea, Tofieldia. LILIALES ALSTROEMERIACEAE: Alstroemeria COLCHICACEAE: Colchicum, Uvularia. LILIACEAE: Clintonia, Erythronium, Lilium, Medeola, Prosartes, Streptopus, Tricyrtis, Tulipa. MELANTHIACEAE: Amianthium, Anticlea, Chamaelirium, Helonias, Melanthium, Schoenocaulon, Stenanthium, Veratrum, Toxicoscordion, Trillium, Xerophyllum, Zigadenus. -

The Vascular Plants of Massachusetts
The Vascular Plants of Massachusetts: The Vascular Plants of Massachusetts: A County Checklist • First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Somers Bruce Sorrie and Paul Connolly, Bryan Cullina, Melissa Dow Revision • First A County Checklist Plants of Massachusetts: Vascular The A County Checklist First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Massachusetts Natural Heritage & Endangered Species Program Massachusetts Division of Fisheries and Wildlife Natural Heritage & Endangered Species Program The Natural Heritage & Endangered Species Program (NHESP), part of the Massachusetts Division of Fisheries and Wildlife, is one of the programs forming the Natural Heritage network. NHESP is responsible for the conservation and protection of hundreds of species that are not hunted, fished, trapped, or commercially harvested in the state. The Program's highest priority is protecting the 176 species of vertebrate and invertebrate animals and 259 species of native plants that are officially listed as Endangered, Threatened or of Special Concern in Massachusetts. Endangered species conservation in Massachusetts depends on you! A major source of funding for the protection of rare and endangered species comes from voluntary donations on state income tax forms. Contributions go to the Natural Heritage & Endangered Species Fund, which provides a portion of the operating budget for the Natural Heritage & Endangered Species Program. NHESP protects rare species through biological inventory, -

Physiological Features of the Terrestrial Orchids
Anallelle Uniiversiităţiiii diin Craiiova, seriia Agriiculltură – Montanollogiie – Cadastru (Annalls of the Uniiversiity of Craiiova - Agriicullture, Montanollogy, Cadastre Seriies)Voll. XLIX/2019 PHYSIOLOGICAL FEATURES OF THE TERRESTRIAL ORCHIDS CEPHALANTHERA LONGIFOLIA AND PLATANTHERA BIFOLIA THAT GROW IN THE PEDO-CLIMATIC CONDITIONS FROM OLTENIA REGION OF ROMANIA BUSE-DRAGOMIR LUMINITA (1), NICOLAE ION (2), NICULESCU MARIANA (3) (1) University of Craiova, E-mail: [email protected] (2) University of Craiova, E-mail: [email protected] (3) University of Craiova, E-mail: [email protected] *Corresponding author email: [email protected] Key words: terrestrial orchids, transpiration, photosynthesis, light ABSTRACT For studying the physiology of terrestrial orchids, plants from the species Cephalanthera longifolia and Platanthera bifolia have been used. The experiences were carried out directly on the field, in Mehedinţi County, Comanesti Hills. In both species of orchids, the seasonal dynamics of photosynthesis registers a peak during the flowering period that corresponds to a maximum content of assimilating pigments and also a maximum leaf surface. In terrestrial orchids, the seasonal dynamics of leaf transpiration intensity is highest during spring, when the water content in the soil is high and minimal in summer. The graphs showing the diurnal variation of photosynthesis for the two species indicate that Cephalanthera longifolia prefers semi-shaded and sunny habitats, while the plants of Platanthera bifolia that are found in the studied areas prefer a more shaded environment INTRODUCTION Orchids that grow directly at the two, more or less globular or digitally- ground level in large areas of Europe or branched tubules. From the main tuber on the grasslands of the tropical regions comes the flowering stem. -

Bletilla Striata (Orchidaceae) Seed Coat Restricts the Invasion of Fungal Hyphae at the Initial Stage of Fungal Colonization
plants Article Bletilla striata (Orchidaceae) Seed Coat Restricts the Invasion of Fungal Hyphae at the Initial Stage of Fungal Colonization Chihiro Miura 1, Miharu Saisho 1, Takahiro Yagame 2, Masahide Yamato 3 and Hironori Kaminaka 1,* 1 Faculty of Agriculture, Tottori University, 4-101 Koyama Minami, Tottori 680-8553, Japan 2 Mizuho Kyo-do Museum, 316-5 Komagatafujiyama, Mizuho, Tokyo 190-1202, Japan 3 Faculty of Education, Chiba University, 1-33 Yayoicho, Inage-ku, Chiba 263-8522, Japan * Correspondence: [email protected]; Tel.: +81-857-31-5378 Received: 24 June 2019; Accepted: 8 August 2019; Published: 11 August 2019 Abstract: Orchids produce minute seeds that contain limited or no endosperm, and they must form an association with symbiotic fungi to obtain nutrients during germination and subsequent seedling growth under natural conditions. Orchids need to select an appropriate fungus among diverse soil fungi at the germination stage. However, there is limited understanding of the process by which orchids recruit fungal associates and initiate the symbiotic interaction. This study aimed to better understand this process by focusing on the seed coat, the first point of fungal attachment. Bletilla striata seeds, some with the seed coat removed, were prepared and sown with symbiotic fungi or with pathogenic fungi. The seed coat-stripped seeds inoculated with the symbiotic fungi showed a lower germination rate than the intact seeds, and proliferated fungal hyphae were observed inside and around the stripped seeds. Inoculation with the pathogenic fungi increased the infection rate in the seed coat-stripped seeds. The pathogenic fungal hyphae were arrested at the suspensor side of the intact seeds, whereas the seed coat-stripped seeds were subjected to severe infestation. -

Phylogenetic Relationships of Discyphus Scopulariae
Phytotaxa 173 (2): 127–139 ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ PHYTOTAXA Copyright © 2014 Magnolia Press Article ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.173.2.3 Phylogenetic relationships of Discyphus scopulariae (Orchidaceae, Cranichideae) inferred from plastid and nuclear DNA sequences: evidence supporting recognition of a new subtribe, Discyphinae GERARDO A. SALAZAR1, CÁSSIO VAN DEN BERG2 & ALEX POPOVKIN3 1Departamento de Botánica, Instituto de Biología, Universidad Nacional Autónoma de México, Apartado Postal 70-367, 04510 México, Distrito Federal, México; E-mail: [email protected] 2Universidade Estadual de Feira de Santana, Departamento de Ciências Biológicas, Av. Transnordestina s.n., 44036-900, Feira de Santana, Bahia, Brazil 3Fazenda Rio do Negro, Entre Rios, Bahia, Brazil Abstract The monospecific genus Discyphus, previously considered a member of Spiranthinae (Orchidoideae: Cranichideae), displays both vegetative and floral morphological peculiarities that are out of place in that subtribe. These include a single, sessile, cordate leaf that clasps the base of the inflorescence and lies flat on the substrate, petals that are long-decurrent on the column, labellum margins free from sides of the column and a column provided with two separate, cup-shaped stigmatic areas. Because of its morphological uniqueness, the phylogenetic relationships of Discyphus have been considered obscure. In this study, we analyse nucleotide sequences of plastid and nuclear DNA under maximum parsimony -

Endophytic Colletotrichum Species from Bletilla Ochracea (Orchidaceae), with Descriptions of Seven New Speices
Fungal Diversity (2013) 61:139–164 DOI 10.1007/s13225-013-0254-5 Endophytic Colletotrichum species from Bletilla ochracea (Orchidaceae), with descriptions of seven new speices Gang Tao & Zuo-Yi Liu & Fang Liu & Ya-Hui Gao & Lei Cai Received: 20 May 2013 /Accepted: 1 July 2013 /Published online: 19 July 2013 # Mushroom Research Foundation 2013 Abstract Thirty-six strains of endophytic Colletotrichum ornamental plants and important research materials for coevo- species were isolated from leaves of Bletilla ochracea Schltr. lution between plants and fungi because of their special sym- (Orchidaceae) collected from 5 sites in Guizhou, China. biosis with mycorrhizal fungi (Zettler et al. 2004; Stark et al. Seventeen different species, including 7 new species (namely 2009; Nontachaiyapoom et al. 2010). Recently, the fungal C. bletillum, C. caudasporum, C. duyunensis, C. endophytum, communities in leaves and roots of orchid Bletilla ochracea C. excelsum-altitudum and C. guizhouensis and C. ochracea), have been investigated and the results indicated that there is a 8 previously described species (C. boninense, C. cereale, C. high diversity of endophytic fungi, including species from the destructivum, C. karstii, C. liriopes, C. miscanthi, C. genus Colletotrichum Corda (Tao et al. 2008, 2012). parsonsiae and C. tofieldiae) and 2 sterile mycelia were iden- Endophytic fungi live asymptomatically and internally with- tified. All of the taxa were identified based on morphology and in different tissues (e.g. leaves, roots) of host plants (Ganley phylogeny inferred from multi-locus sequences, including the and Newcombe 2006; Promputtha et al. 2007; Hoffman and nuclear ribosomal internal transcribed spacer (ITS) region, Arnold 2008). -

Aphyllorchis Queenslandica Click on Images to Enlarge
Species information Abo ut Reso urces Hom e A B C D E F G H I J K L M N O P Q R S T U V W X Y Z Aphyllorchis queenslandica Click on images to enlarge Family Orchidaceae Scientific Name Aphyllorchis queenslandica Dockrill Dockrill, A.W. (1965) Orchadian 1: 115. Type: Queensland, Helenvale, May 1962, C. Le Roy: Holo: QRS. Common name Herbarium specimen. Copyright CSIRO Yellow Pauper Orchid Stem Above ground part of the plant (peduncle + inflorescence) about 65-90 cm tall. Leaves Plant devoid of chlorophyll. 'Leaves' (bracts) about 8-10 per plant, sessile, about 0.5-3 x 0.6-1.1 cm. Bracts 3-veined, venation longitudinal and parallel. Flowers Sepals about 13 x 2.5 mm. Petals about 11 x 2 mm, labellum larger. Labellum cream, margins upturned, purple. Column purple at the base, but yellow towards the apex. Stamen fused to the style to form a column. Staminal column about 7 x 1.5 mm. Ovary about 12 x 2 mm, outer surface with 6 longitudinal ribs. Fruit Inflorescence about 40-70 cm long, usually 6-12-flowered. Peduncle with up to 10 stem-clasping bracts, each bract about 5-20 x 6-10 mm. Bracts subtending the flowers about 10 x 2 mm and are not stem-clasping. Flowers about 20 mm wide on pedicels about 2-3 mm long. Sepals about 11-13 c 3-4 mm. Petals about 11 x 3 mm. Labellum about 6 x 2.5 mm. Column about 6-8 x 1.5 mm, curved, semi-cylindrical. -
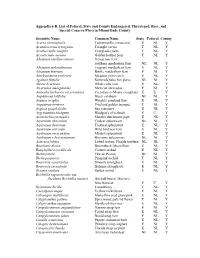
Conservation Appendix 6-B Listed Flora
Appendix 6-B. List of Federal, State and County Endangered, Threatened, Rare, and Special Concern Flora in Miami-Dade County Scientific Name Common Name State Federal County Acacia choriophylla Tamarindillo; cinnecord E NL Y Acanthocereus tetragenus Triangle cactus T NL Y Acoelorraphe wrightii Everglades palm T NL Y Acrostichum aureum Golden leather fern T NL Y Adiantum capillus-veneris Venus hair fern; southern maidenhair fern NL NL Y Adiantum melanoleucum Fragrant maidenhair fern E NL Y Adiantum tenerum Brittle maidenhair fern E NL Y Aeschynomene pratensis Meadow joint-vetch E NL Y Agalinis filifolia Seminole false fox glove NL NL Y Aletris bracteata White colic root E NL Y Alvaradoa amorphoides Mexican alvaradoa E NL Y Amorpha herbacea var.crenulata Crenulate (=Miami) leadplant E E Y Amphitecna latifolia Black calabash NL NL Y Anemia wrightii Wright's pineland fern E NL Y Angadenia berteroi Pineland golden trumpet T NL Y Argusia gnaphalodes Sea rosemary E NL Y Argythamnia blodgettii Blodgett's silverbush E C Y Aristolochia pentandra Marsh's dutchmans pipe E NL Y Asplenium abscissum Cutleaf spleenwort NL NL Y Asplenium dentatum Toothed spleenwort E NL Y Asplenium serratum Wild bird nest fern E NL Y Asplenium verecundum Modest spleenwort E NL Y Asplenium x biscaynianum Biscayne spleenwort NL NL Y Asteraea lobata Lobed croton; Florida treefern NL NL Y Baccharis dioica Broombush falsewillow E NL Y Basiphyllaea corallicola Carter's orchid E NL Y Bletia patula Flor de Pesmo NL NL Y Bletia purpurea Pinepink orchid T NL Y Bourreria cassinifolia Smooth strongback E NL Y Bourreria succulenta Bahama strongback E NL Y Brassia caudata Spider orchid E NL Y Brickellia eupatorioides var. -

Redalyc.EFFORTS to CONSERVE ENDANGERED TERRESTRIAL
Lankesteriana International Journal on Orchidology ISSN: 1409-3871 [email protected] Universidad de Costa Rica Costa Rica RANGEL-VILLAFRANCO, MONICA; ORTEGA-LARROCEA, M. PILAR EFFORTS TO CONSERVE ENDANGERED TERRESTRIAL ORCHIDS IN SITU AND EX SITU AT TWO NATURAL RESERVES WITHIN CENTRAL MEXICO Lankesteriana International Journal on Orchidology, vol. 7, núm. 1-2, marzo, 2007, pp. 326-333 Universidad de Costa Rica Cartago, Costa Rica Available in: http://www.redalyc.org/articulo.oa?id=44339813068 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative LANKESTERIANA 7(1-2): 326-333. 2007. EFFORTS TO CONSERVE ENDANGERED TERRESTRIAL ORCHIDS IN SITU AND EX SITU AT TWO NATURAL RESERVES WITHIN CENTRAL MEXICO 1 1,2 MONICA RANGEL-VILLAFRANCO & M. PILAR ORTEGA-LARROCEA 1 Departamento de Edafología, Instituto de Geología, Universidad Nacional Autónoma de México. Circuito Exterior de Ciudad Universitaria, México Distrito Federal, 04510. México. 2 Author for correspondence: [email protected] KEY WORDS: in situ conservation, ex situ conservation, orchid fungi isolation, seed banks The natural vegetation in and around Mexico City macrobulon, Epidendrum anisatu, Habenaria strictis- once harbored an unusually high number of plant and sima, Liparis greenwoodiana) (Hágsater et al. 2005). animal (insect) species, including endemics (Vázquez In contrast, in the Chichinautzin Area, eight types 1973, Ceballos & Galindo 1984, Rzedowski 1991). of vegetation can be found. An altitudinal gradient The high diversity in this region has been attributed joint with successive periods of volcanic activity to the unusual topography resulting from a series of are combined and pedogenetic processes through volcanic eruptions that ended ca.