Phytogeographical Analysis and Ecological Factors of the Distribution of Orchidaceae Taxa in the Western Carpathians (Local Study)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![Pollinator Diversity and Reproductive Success of [I]Epipactis Helleborine[I]](https://docslib.b-cdn.net/cover/3678/pollinator-diversity-and-reproductive-success-of-i-epipactis-helleborine-i-43678.webp)
Pollinator Diversity and Reproductive Success of [I]Epipactis Helleborine[I]
Manuscript to be reviewed Pollinator diversity and reproductive success of Epipactis helleborine (L.) Crantz (Orchidaceae) in anthropogenic and natural habitats Agnieszka Rewicz Corresp., 1 , Radomir Jaskuła 2 , Tomasz Rewicz 3 , Grzegorz Tończyk 2 1 Department of Geobotany and Plant Ecology, University of Lodz, Łódź, Poland 2 Department of Invertebrate Zoology and Hydrobiology, University of Lodz, Łódź, Poland 3 Laboratory of Microscopic Imaging and Specialized Biological Techniques, University of Lodz, Łódź, Poland Corresponding Author: Agnieszka Rewicz Email address: [email protected] Background. Epipactis helleborine is an Eurasian orchid species which prefers woodland environments but it may also spontaneously and successfully colonise human-made artificial and disturbed habitats such as roadsides, town parks and gardens. It is suggested that orchids colonising anthropogenic habitats are characterised by a specific set of features (e.g.: large plant size, fast flower production). However, as it is not well known how pollinator diversity and reproductive success of E. helleborine differs in populations in anthropogenic habitats compared to populations from natural habitats, we wanted to compare pollinator diversity and reproductive success of this orchid species between natural and anthropogenic habitat types. Methods. Pollination biology, reproductive success and autogamy in populations of E. helleborine from anthropogenic (roadside) and natural (forest) habitats were compared. Eight populations (four natural and four human-disturbed ones) in two seasons were studied according to height of plants, length of inflorescences, as well as numbers of juvenile shoots, flowering shoots, flowers, and fruits. The number and diversity of insect pollinators were studied in one natural and two human-disturbed populations. Results. -

November December 2010 Vol 67.3 Victoria Natural
NOVEMBER DECEMBER 2010 VOL 67.3 VICTORIA NATURAL HISTORY SOCIETY The Victoria Naturalist Vol. 67.3 (2010) 1 Published six times a year by the SUBMISSIONS VICTORIA NATURAL HISTORY SOCIETY, P.O. Box 5220, Station B, Victoria, BC V8R 6N4 Deadline for next issue: December 1, 2010 Contents © 2010 as credited. Send to: Claudia Copley ISSN 0049—612X Printed in Canada 657 Beaver Lake Road, Victoria BC V8Z 5N9 Editors: Claudia Copley, 250-479-6622, Penelope Edwards Phone: 250-479-6622 Desktop Publishing: Frances Hunter, 250-479-1956 e-mail: [email protected] Distribution: Tom Gillespie, Phyllis Henderson, Morwyn Marshall Printing: Fotoprint, 250-382-8218 Guidelines for Submissions Opinions expressed by contributors to The Victoria Naturalist Members are encouraged to submit articles, field trip reports, natural are not necessarily those of the Society. history notes, and book reviews with photographs or illustrations if possible. Photographs of natural history are appreciated along with VICTORIA NATURAL HISTORY SOCIETY documentation of location, species names and a date. Please label your Honorary Life Members Dr. Bill Austin, Mrs. Lyndis Davis, submission with your name, address, and phone number and provide a Mr. Tony Embleton, Mr. Tom Gillespie, Mrs. Peggy Goodwill, title. We request submission of typed, double-spaced copy in an IBM compatible word processing file on diskette, or by e-mail. Photos and Mr. David Stirling, Mr. Bruce Whittington slides, and diskettes submitted will be returned if a stamped, self- Officers: 2009-2010 addressed envelope is included with the material. Digital images are PRESIDENT: Darren Copley, 250-479-6622, [email protected] welcome, but they need to be high resolution: a minimum of 1200 x VICE-PRESIDENT: James Miskelly, 250-477-0490, [email protected] 1550 pixels, or 300 dpi at the size of photos in the magazine. -

Guide to the Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- LILIACEAE
Guide to the Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- LILIACEAE LILIACEAE de Jussieu 1789 (Lily Family) (also see AGAVACEAE, ALLIACEAE, ALSTROEMERIACEAE, AMARYLLIDACEAE, ASPARAGACEAE, COLCHICACEAE, HEMEROCALLIDACEAE, HOSTACEAE, HYACINTHACEAE, HYPOXIDACEAE, MELANTHIACEAE, NARTHECIACEAE, RUSCACEAE, SMILACACEAE, THEMIDACEAE, TOFIELDIACEAE) As here interpreted narrowly, the Liliaceae constitutes about 11 genera and 550 species, of the Northern Hemisphere. There has been much recent investigation and re-interpretation of evidence regarding the upper-level taxonomy of the Liliales, with strong suggestions that the broad Liliaceae recognized by Cronquist (1981) is artificial and polyphyletic. Cronquist (1993) himself concurs, at least to a degree: "we still await a comprehensive reorganization of the lilies into several families more comparable to other recognized families of angiosperms." Dahlgren & Clifford (1982) and Dahlgren, Clifford, & Yeo (1985) synthesized an early phase in the modern revolution of monocot taxonomy. Since then, additional research, especially molecular (Duvall et al. 1993, Chase et al. 1993, Bogler & Simpson 1995, and many others), has strongly validated the general lines (and many details) of Dahlgren's arrangement. The most recent synthesis (Kubitzki 1998a) is followed as the basis for familial and generic taxonomy of the lilies and their relatives (see summary below). References: Angiosperm Phylogeny Group (1998, 2003); Tamura in Kubitzki (1998a). Our “liliaceous” genera (members of orders placed in the Lilianae) are therefore divided as shown below, largely following Kubitzki (1998a) and some more recent molecular analyses. ALISMATALES TOFIELDIACEAE: Pleea, Tofieldia. LILIALES ALSTROEMERIACEAE: Alstroemeria COLCHICACEAE: Colchicum, Uvularia. LILIACEAE: Clintonia, Erythronium, Lilium, Medeola, Prosartes, Streptopus, Tricyrtis, Tulipa. MELANTHIACEAE: Amianthium, Anticlea, Chamaelirium, Helonias, Melanthium, Schoenocaulon, Stenanthium, Veratrum, Toxicoscordion, Trillium, Xerophyllum, Zigadenus. -

Physiological Features of the Terrestrial Orchids
Anallelle Uniiversiităţiiii diin Craiiova, seriia Agriiculltură – Montanollogiie – Cadastru (Annalls of the Uniiversiity of Craiiova - Agriicullture, Montanollogy, Cadastre Seriies)Voll. XLIX/2019 PHYSIOLOGICAL FEATURES OF THE TERRESTRIAL ORCHIDS CEPHALANTHERA LONGIFOLIA AND PLATANTHERA BIFOLIA THAT GROW IN THE PEDO-CLIMATIC CONDITIONS FROM OLTENIA REGION OF ROMANIA BUSE-DRAGOMIR LUMINITA (1), NICOLAE ION (2), NICULESCU MARIANA (3) (1) University of Craiova, E-mail: [email protected] (2) University of Craiova, E-mail: [email protected] (3) University of Craiova, E-mail: [email protected] *Corresponding author email: [email protected] Key words: terrestrial orchids, transpiration, photosynthesis, light ABSTRACT For studying the physiology of terrestrial orchids, plants from the species Cephalanthera longifolia and Platanthera bifolia have been used. The experiences were carried out directly on the field, in Mehedinţi County, Comanesti Hills. In both species of orchids, the seasonal dynamics of photosynthesis registers a peak during the flowering period that corresponds to a maximum content of assimilating pigments and also a maximum leaf surface. In terrestrial orchids, the seasonal dynamics of leaf transpiration intensity is highest during spring, when the water content in the soil is high and minimal in summer. The graphs showing the diurnal variation of photosynthesis for the two species indicate that Cephalanthera longifolia prefers semi-shaded and sunny habitats, while the plants of Platanthera bifolia that are found in the studied areas prefer a more shaded environment INTRODUCTION Orchids that grow directly at the two, more or less globular or digitally- ground level in large areas of Europe or branched tubules. From the main tuber on the grasslands of the tropical regions comes the flowering stem. -

The Genomic Impact of Mycoheterotrophy in Orchids
fpls-12-632033 June 8, 2021 Time: 12:45 # 1 ORIGINAL RESEARCH published: 09 June 2021 doi: 10.3389/fpls.2021.632033 The Genomic Impact of Mycoheterotrophy in Orchids Marcin J ˛akalski1, Julita Minasiewicz1, José Caius2,3, Michał May1, Marc-André Selosse1,4† and Etienne Delannoy2,3*† 1 Department of Plant Taxonomy and Nature Conservation, Faculty of Biology, University of Gdansk,´ Gdansk,´ Poland, 2 Institute of Plant Sciences Paris-Saclay, Université Paris-Saclay, CNRS, INRAE, Univ Evry, Orsay, France, 3 Université de Paris, CNRS, INRAE, Institute of Plant Sciences Paris-Saclay, Orsay, France, 4 Sorbonne Université, CNRS, EPHE, Muséum National d’Histoire Naturelle, Institut de Systématique, Evolution, Biodiversité, Paris, France Mycoheterotrophic plants have lost the ability to photosynthesize and obtain essential mineral and organic nutrients from associated soil fungi. Despite involving radical changes in life history traits and ecological requirements, the transition from autotrophy Edited by: Susann Wicke, to mycoheterotrophy has occurred independently in many major lineages of land Humboldt University of Berlin, plants, most frequently in Orchidaceae. Yet the molecular mechanisms underlying this Germany shift are still poorly understood. A comparison of the transcriptomes of Epipogium Reviewed by: Maria D. Logacheva, aphyllum and Neottia nidus-avis, two completely mycoheterotrophic orchids, to other Skolkovo Institute of Science autotrophic and mycoheterotrophic orchids showed the unexpected retention of several and Technology, Russia genes associated with photosynthetic activities. In addition to these selected retentions, Sean W. Graham, University of British Columbia, the analysis of their expression profiles showed that many orthologs had inverted Canada underground/aboveground expression ratios compared to autotrophic species. Fatty Craig Barrett, West Virginia University, United States acid and amino acid biosynthesis as well as primary cell wall metabolism were among *Correspondence: the pathways most impacted by this expression reprogramming. -
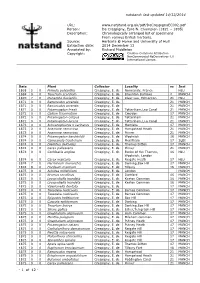
Date Plant Collector Locality Vc Inst 1868 5 0 Primula Polyantha Crespigny, E
natstand: last updated 14/12/2014 URL: www.natstand.org.uk/pdf/DeCrespignyEC002.pdf Person: De Crespigny, Eyre N. Champion (1821 – 1895) Description: Chronologically arranged list of specimens From various British herbaris. Source: Herbaria @ Home and University of Hull Extraction date: 2014 December 13 Annotated by: Richard Middleton Copyright: Creative Commons Attribution- NonCommercial-NoDerivatives 4.0 International License. Date Plant Collector Locality vc Inst 1868 5 0 Primula polyantha Crespigny, E. de Normandy, France HLU 1869 0 0 Teucrium scordium Crespigny, E. de Braunton Burrows 4 MANCH 1870 7 0 Oenanthe fluviatilis Crespigny, E. de River Lee, Edmonton 21 HLU 1871 0 0 Ranunculus arvensis Crespigny, E. de 21 MANCH 1871 0 0 Ranunculus arvensis Crespigny, E. de 21 MANCH 1871 0 0 Potamogeton friesii Crespigny, E. de Tottenham,Lea Canal 21 MANCH 1872 0 0 Galium tricornutum Crespigny, E. de Croydon 17 MANCH 1872 0 0 Potamogeton crispus Crespigny, E. de Tottenham 21 MANCH 1872 0 0 Potamogeton lucens Crespigny, E. de Tottenham,Lea Canal 21 MANCH 1873 0 0 Schoenoplectus x carinatus Crespigny, E. de Mortlake 17 MANCH 1873 0 0 Anemone nemorosa Crespigny, E. de Hampstead Heath 21 MANCH 1873 0 0 Anemone nemorosa Crespigny, E. de Pinner 21 MANCH 1874 0 0 Potamogeton berchtoldii Crespigny, E. de Woolwich 16 MANCH 1874 0 0 Campanula trachelium Crespigny, E. de Merstham 17 SLBI 1874 0 0 Dianthus deltoides Crespigny, E. de Thames Ditton 17 MANCH 1874 0 0 Carex pallescens Crespigny, E. de Pinner 21 MANCH 1874 0 0 Cochlearia anglica Crespigny, E. de Banks of the Thames, 16 HLU Woolwich, London 1874 6 0 Carex vesicaria Crespigny, E. -

Taxonomic Notes on Anacamptis Pyramidalis Var. Urvilleana (Orchidaceae), a Good Endemic Orchid from Malta
J. Eur. Orch. 48 (1): 19 – 28. 2016. Stephen Mifsud Taxonomic notes on Anacamptis pyramidalis var. urvilleana (Orchidaceae), a good endemic orchid from Malta Keywords Orchidaceae; Anacamptis urvilleana; Anacamptis pyramidalis; Anacamptis pyramidalis var. urvilleana; Maltese endemics; Flora of Malta; Central Mediterranean region. Summary Mifsud S. (2016): Taxonomic notes on Anacamptis pyramidalis var. urvilleana (Orchidaceae), a good endemic orchid from Malta.- J. Eur. Orch. 48 (1): 19-28. In several global plant species databases the Maltese-endemic Anacamptis urvilleana is considered as a synonym of A. pyramidalis, hence reflecting the belief of some European authors. A number of morphological differences and phenology differentiate the Maltese pyramidical orchid from A. pyramidalis. As a result, it is suggested to maintain the identity of this orchid as A. pyramidalis var. urvilleana which merits conservation treatments different from the widely distributed A. pyramidalis s. str. Zusammenfassung Mifsud S. (2016): Taxonomische Anmerkungen zu Anacamptis pyramidalis var. urvilleana (Orchidaceae), eine gute endemische Orchidee von Malta.- J. Eur. Orch. 48 (1): 19-28. In verschiedenen weltweiten Datenbanken botanischer Namen, die auch die Meinung einiger europäischer Autoren wiedergeben, wird der maltesische Endemit Anacamptis urvilleana als Synonym von A. pyramidalis geführt. Die maltesische Pyramiden-Hundswurz unterscheidet sich jedoch sowohl in einer Reihe von morphologischen Merkmalen als auch phenologisch von A. pyramidalis. Auf dieser Grundlage wird vorgeschlagen, diese Orchidee als A. pyramidalis var. urvilleana zu führen. Zu ihrem Schutz sind andere Erhaltungsmaßnahmen erforderlich als für die weitverbreitete A. pyramidalis s. str. Journal Europäischer Orchideen 48 (1): 2016. 19 1. Introduction Anacamptis urvilleana Sommier & Caruana Gatto was described in 1915 (refer Fig.1) as an endemic orchid from the Maltese islands. -

Watsonia 14 (1982), 79-100
Walsonia , 14, 79-100 (\982) 79 Book Reviews Flowers of Greece and the Balkans. A field guide. Oleg Polunin. Pp. xv+592 including 62 pages of line drawings and 21 maps, with 80 colour plates. Oxford University Press, Oxford. 1980. Price £40.00 (ISBN 0-19- 217-6269). This latest of Oleg Polunin's Field guides to the European flora presents a concise, but by no means superficial, picture of the flowering plants and conifers of the Balkan peninsula. The area that is covers takes in the whole of Greece (including the East Aegean Islands, excluded from Flora Europaea), Turkey-in-Europe, Albania, Bulgaria, Jugoslavia as far north as the River Sava, and the small portion of Romania that lies south and east of the River Danube. The author has condensed his account of the rich and varied flora of the region, together with the associated mass of published material, into a form that is attractive, comprehensible and useful to the amateur botanist. A book of this type has been badly needed, as the available floristic texts on the Balkans tend to be over 50 years old, scarce, extremely expensive and written in a foreign language, often Latin. However, the most significant attribute of this book is not that it gives access to diffuse and obscure information, but that it provides a radical alternative to popular botanical accounts of Greece which emphasize the lowland spring flora, notably the orchids and other petaloid monocots. Based on the author's many years of botanical experience and extensive travel in the region, Flowers of Greece and the Balkans demonstrates the wide range of flora and vegetation that the amateur botanist can expect to see in the Balkan peninsula. -

Latvijas Universitātes Zinātniskie Raksti Acta Universitatis Latviensis
ISSN 1407-2157 Latvijas Universitātes Zinātniskie Raksti Acta Universitatis Latviensis 613 LATVIJAS PURVU VEĢETĀCIJAS KLASIFIKĀCIJA UN DINAMIKA Latvijas Universitāte Latvijas purvu veģetācijas klasifikācija un dinamika Zinātniskie raksti 613. sējums Rīga 1998 -) / Latvijas punu veģetācijas klasifikācija un dinamika: Zinātniskie raksti/Redkolēģija: V.Kreile, M.Laiviņš, A.Namatēva. Rīga: LU, 1998. 92 Ipp. Rakstu krājumā apkopoti pēdējo gadu Latvijas purvu un ezeru krastu veģetācijas pētījumu rezultāti. Analizēti Teicu purva veidošanās apstākļi pēc putekšņu diagrammām. Publicētas purvu augu sabiedrību sintaksonomijas shēmas un sinoptiskās tabulas. Pētījumu rezultātus var izmantot bioloģijas un ģeogrāfijas studenti un citi interesenti. Redakcijas kolēģija: Vija Kreile, Māris Laiviņš, Anita Namatēva © Teicu valsts rezervāts, 1998 PRIEKŠVĀRDS 1997.gada 20.-21.oktobri Teicu rezervātā notika seminārs "Purvu veģetācijas klasifikācija, kartēšana un aizsardzība Latvijā", kurā piedalījās Latvijas Universitātes Bioloģijas un Ģeogrāfijas un Zemes zinātņu fakultāšu, Valsts Ģeoloģijas dienesta, Latvijas Valsts Mežzinātnes institūta "Silava" un Teicu valsts rezervāta speciālisti. Latvijas lielākajā purvu masīvā Teicos notika ekspedīcijas semināra dalībnieku iepazīstināšanai ar sūnu purvu ciņu un lāmu, pārejas un zāļu purvu, ezeru aizaugšanas joslu un palienes pļavu veģetāciju 2 maršrutos: Stiebriņi Kurtavas ezers Šūmāna ezers un Silagals Tolkajas ezers Siksala Islienas ezers. Seminārā tika nolasīti 8 ziņojumi par purvu veģetācijas un floras pētījumiem dažādos Latvijas reģionos, demonstrētas kartes un sintaksonomijas shēmas. Šajā rakstu krājumā publicēti semināra materiāli. Semināra norisi un rakstu krājuma sagatavošanu atbalstīja LR Vides aizsardzības fonds un Teicu valsts rezervāts. SATURS M.Laiviņš. Latvijas ziedaugu un paparžaugu sabiedrību augstākie sintaksoni 7 M.Pakalne. Latvijas purvu veģetācijas raksturojums 23 A. Lācis, L.Kalniņa. Purvu uzbūve un attīstība Teicu valsts rezervātā 39 B.Bambe. Purvu veģetācijas dinamika Teicu rezervātā 56 S.Jermacāne. -

Click to View .Pdf of This Issue
JJoouurrnnaall of the HHAARRDDYY OORRCCHHIIDD SSOOCCIIEETTYY Vol. 6 No. 2 (52) April 2009 JOURNAL of the HARDY ORCHID SOCIETY Vol. 6 No. 2 (52) April 2009 The Hardy Orchid Society Our aim is to promote interest in the study of Native European Orchids and those from similar temperate climates throughout the world. We cover such varied aspects as field study, cultivation and propagation, photography, taxonomy and systematics, and practical conservation. We welcome articles relating to any of these subjects, which will be considered for publication by the editorial committee. Please send your submissions to the Editor, and please structure your text according to the “Advice to Authors” (see website, January 2004 Journal, Members’ Handbook or contact the Editor). Views expressed in journal articles are those of their author(s) and may not reflect those of HOS. The Hardy Orchid Society Committee President: Prof. Richard Bateman, Jodrell Laboratory, Royal Botanic Gardens Kew, Richmond, Surrey, TW9 3DS Chairman & Field Meeting Co-ordinator: David Hughes, Linmoor Cottage, Highwood, Ringwood, Hants., BH24 3LE [email protected] Vice-Chairman: Celia Wright, The Windmill, Vennington, Westbury, Shrewsbury, Shropshire, SY5 9RG [email protected] Secretary (Acting): Alan Leck, 61 Fraser Close, Deeping St. James, Peterborough, PE6 8QL [email protected] Treasurer: Iain Wright, The Windmill, Vennington, Westbury, Shrewsbury, Shropshire, SY5 9RG [email protected] Membership Secretary: Celia Wright, The Windmill, Vennington, Westbury, -

Forma Nov.: a New Peloric Orchid from Ibaraki Prefecture, Japan
The Japanese Society for Plant Systematics ISSN 1346-7565 Acta Phytotax. Geobot. 65 (3): 127–139 (2014) Cephalanthera falcata f. conformis (Orchidaceae) forma nov.: A New Peloric Orchid from Ibaraki Prefecture, Japan 1,* 1 1 HirosHi Hayakawa , CHiHiro Hayakawa , yosHinobu kusumoto , tomoko 1 1 2 3,4 nisHida , Hiroaki ikeda , tatsuya Fukuda and Jun yokoyama 1National Institute for Agro-Environmental Sciences, 3-1-3 Kannondai, Tsukuba, Ibaraki 305-8604, Japan. *[email protected] (author for correspondences); 2Faculty of Agriculture, Kochi University, Nan- koku, Kochi 783-8502, Japan; 3Faculty of Science, Yamagata University, Kojirakawa, Yamagata 990-8560, Japan; 4Institute for Regional Innovation, Yamagata University, Kaminoyama, Yamagata 999-3101, Japan We recognize a new peloric form of the orchid Cephalanthera falcata (Thunb.) Blume f. conformis Hi- ros. Hayak. et J. Yokoy., which occurs in the vicinity of the Tsukuba mountain range in Ibaraki Prefec- ture, Japan. This peloric form is sympatric with C. falcata f. falcata. The peloric flowers have a petal-like lip. The flowers are radially symmetrical and the perianth parts spread weakly compared to normal flow- ers. The stigmas are positioned at the column apex, thus neighboring stigmas and the lower parts of the pollinia are conglutinated. We observed similar vegetative traits among C. falcata f. falcata, C. falcata f. albescens S. Kobayashi, and C. falcata f. conformis. The floral morphology in C. falcata f. conformis resembles that of C. nanchuanica (S.C. Chen) X.H. Jin et X.G. Xiang (i.e., similar petal-like lip and stig- ma position at the column apex; syn. Tangtsinia nanchuanica S.C. -

Rare Plant Monitoring 2017
RARE PLANT MONITORING 2017 Ajuga pyramidalis Ophrys insectifera © Zoe Devlin What is it? In 2017, we decided to carry out a small pilot scheme on rare plant monitoring. Where experienced plant recorders had submitted recent casual records of rare plants to the Centre, they were asked if they would be willing to visit their rare plant population once a year during its flowering period and to count the total number of individuals present. The response to the scheme from the small number of recorders contacted has been overwhelming positive and it has resulted in very valuable data being collected in 2017. Data on the rare plant location, the count and additional information about the site is submitted online through a dedicated web portal set up by the Data Centre. The project was discussed and agreed with the NPWS. It is framed around the 2016 Vascular Plant Red List and is mainly focused on monitoring vulnerable, near threatened and rare least concern species. Why is it important? When assessing the national FAST FACTS 2017 conservation status of very rare species according to IUCN Red List methodology, it is recommended that 37 you use annual population count data. That’s the total number of rare plant Given the numbers of rare plant populations that were monitored in the species a country might have, this 2017 pilot information can be difficult to collect in any volume. This citizen science project relies on the generosity of 22 expert volunteers to ‘keep an eye’ on That’s the number of rare plant species rare populations near them and to that were monitored in 2017 submit standardised count data once a year.