Forma Nov.: a New Peloric Orchid from Ibaraki Prefecture, Japan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Comparative Study on the Reproductive Success of Two Rewarding Habenaria Species
Zhang and Gao BMC Plant Biology (2021) 21:187 https://doi.org/10.1186/s12870-021-02968-w RESEARCH ARTICLE Open Access A comparative study on the reproductive success of two rewarding Habenaria species (Orchidaceae) occurring in roadside verge habitats Wenliu Zhang1,2 and Jiangyun Gao1,2* Abstract Background: Most orchid species have been shown to be severely pollination limited, and the factors affecting reproductive success have been widely studied. However, the factors determining the reproductive success vary from species to species. Habenaria species typically produce nectar but exhibit variable fruit set and reproductive success among species. Here, we investigated the influence of the flowering plant density, inflorescence size, breeding system, and pollinator behaviour on the reproductive success of two rewarding Habenaria species. Results: Our observations indicated that Habenaria limprichtii and H. petelotii co-occur in roadside verge habitats and present overlapping flowering periods. Both species were pollination limited, although H. limprichtii produced more fruits than H. petelotii under natural conditions during the 3-year investigation. H. petelotii individuals formed distinct patches along roadsides, while nearly all H. limprichtii individuals clustered together. The bigger floral display and higher nectar sugar concentration in H. limprichtii resulted in increased attraction and visits from pollinators. Three species of effective moths pollinated for H. limprichtii, while Thinopteryx delectans (Geometridae) was the exclusive pollinator of H. petelotii. The percentage of viable seeds was significantly lower for hand geitonogamy than for hand cross-pollination in both species. However, H. limprichtii may often be geitonogamously pollinated based on the behaviours of the pollinators and viable embryo assessment. -

November December 2010 Vol 67.3 Victoria Natural
NOVEMBER DECEMBER 2010 VOL 67.3 VICTORIA NATURAL HISTORY SOCIETY The Victoria Naturalist Vol. 67.3 (2010) 1 Published six times a year by the SUBMISSIONS VICTORIA NATURAL HISTORY SOCIETY, P.O. Box 5220, Station B, Victoria, BC V8R 6N4 Deadline for next issue: December 1, 2010 Contents © 2010 as credited. Send to: Claudia Copley ISSN 0049—612X Printed in Canada 657 Beaver Lake Road, Victoria BC V8Z 5N9 Editors: Claudia Copley, 250-479-6622, Penelope Edwards Phone: 250-479-6622 Desktop Publishing: Frances Hunter, 250-479-1956 e-mail: [email protected] Distribution: Tom Gillespie, Phyllis Henderson, Morwyn Marshall Printing: Fotoprint, 250-382-8218 Guidelines for Submissions Opinions expressed by contributors to The Victoria Naturalist Members are encouraged to submit articles, field trip reports, natural are not necessarily those of the Society. history notes, and book reviews with photographs or illustrations if possible. Photographs of natural history are appreciated along with VICTORIA NATURAL HISTORY SOCIETY documentation of location, species names and a date. Please label your Honorary Life Members Dr. Bill Austin, Mrs. Lyndis Davis, submission with your name, address, and phone number and provide a Mr. Tony Embleton, Mr. Tom Gillespie, Mrs. Peggy Goodwill, title. We request submission of typed, double-spaced copy in an IBM compatible word processing file on diskette, or by e-mail. Photos and Mr. David Stirling, Mr. Bruce Whittington slides, and diskettes submitted will be returned if a stamped, self- Officers: 2009-2010 addressed envelope is included with the material. Digital images are PRESIDENT: Darren Copley, 250-479-6622, [email protected] welcome, but they need to be high resolution: a minimum of 1200 x VICE-PRESIDENT: James Miskelly, 250-477-0490, [email protected] 1550 pixels, or 300 dpi at the size of photos in the magazine. -

IRG Has Articles on Plant Naming, Trough Planting and a Charming Oriental Orchid
International Rock Gardener ISSN 2053-7557 Number 59 The Scottish Rock Garden Club November 2014 ---International Rock Gardener--- November 2014 This month the IRG has articles on plant naming, trough planting and a charming oriental orchid. If you have a favourite plant genus you’d like to discuss, innovative ideas in cultivation, or some other idea about the world of plants and gardens that is important to you, you are most welcome to contact the IRG Team about it. You can make contact via [email protected] – we look forward to hearing from you. If you enjoy reading the IRG each month – and the other resources provided by the Scottish Rock Garden Club on www.srgc.net – we will be most grateful if you choose to show that appreciation of our efforts by making a donation to the work of the SRGC via the “donate” button on any page of the website. IRG Index: A link to a regularly updated index to the IRG can be found here in the SRGC Forum. Cover picture: Crocus vaclavii, photo by Jānis Rukšāns ---Plant Portrait--- Ponerorchis graminifolia text and photos by Grahame Ware, Canada This hardy to Zone 7/8 member of the Orchid family is native to S. Korea and Japan (Honshu, Shikoku and Kyushu). It was authored and named in 1852 by the German botanist Henrich Gustav Reichenbach (1824-1889). It has been officially classed in the past as Orchis as well as Gymnadenia. But ever since Maekawa in 1971 with the publication of his beautifully illustrated Wild Orchids of Japan in Colour, the name Ponerorchis has held sway and continues to do so to this day. -

Physiological Features of the Terrestrial Orchids
Anallelle Uniiversiităţiiii diin Craiiova, seriia Agriiculltură – Montanollogiie – Cadastru (Annalls of the Uniiversiity of Craiiova - Agriicullture, Montanollogy, Cadastre Seriies)Voll. XLIX/2019 PHYSIOLOGICAL FEATURES OF THE TERRESTRIAL ORCHIDS CEPHALANTHERA LONGIFOLIA AND PLATANTHERA BIFOLIA THAT GROW IN THE PEDO-CLIMATIC CONDITIONS FROM OLTENIA REGION OF ROMANIA BUSE-DRAGOMIR LUMINITA (1), NICOLAE ION (2), NICULESCU MARIANA (3) (1) University of Craiova, E-mail: [email protected] (2) University of Craiova, E-mail: [email protected] (3) University of Craiova, E-mail: [email protected] *Corresponding author email: [email protected] Key words: terrestrial orchids, transpiration, photosynthesis, light ABSTRACT For studying the physiology of terrestrial orchids, plants from the species Cephalanthera longifolia and Platanthera bifolia have been used. The experiences were carried out directly on the field, in Mehedinţi County, Comanesti Hills. In both species of orchids, the seasonal dynamics of photosynthesis registers a peak during the flowering period that corresponds to a maximum content of assimilating pigments and also a maximum leaf surface. In terrestrial orchids, the seasonal dynamics of leaf transpiration intensity is highest during spring, when the water content in the soil is high and minimal in summer. The graphs showing the diurnal variation of photosynthesis for the two species indicate that Cephalanthera longifolia prefers semi-shaded and sunny habitats, while the plants of Platanthera bifolia that are found in the studied areas prefer a more shaded environment INTRODUCTION Orchids that grow directly at the two, more or less globular or digitally- ground level in large areas of Europe or branched tubules. From the main tuber on the grasslands of the tropical regions comes the flowering stem. -

The Genomic Impact of Mycoheterotrophy in Orchids
fpls-12-632033 June 8, 2021 Time: 12:45 # 1 ORIGINAL RESEARCH published: 09 June 2021 doi: 10.3389/fpls.2021.632033 The Genomic Impact of Mycoheterotrophy in Orchids Marcin J ˛akalski1, Julita Minasiewicz1, José Caius2,3, Michał May1, Marc-André Selosse1,4† and Etienne Delannoy2,3*† 1 Department of Plant Taxonomy and Nature Conservation, Faculty of Biology, University of Gdansk,´ Gdansk,´ Poland, 2 Institute of Plant Sciences Paris-Saclay, Université Paris-Saclay, CNRS, INRAE, Univ Evry, Orsay, France, 3 Université de Paris, CNRS, INRAE, Institute of Plant Sciences Paris-Saclay, Orsay, France, 4 Sorbonne Université, CNRS, EPHE, Muséum National d’Histoire Naturelle, Institut de Systématique, Evolution, Biodiversité, Paris, France Mycoheterotrophic plants have lost the ability to photosynthesize and obtain essential mineral and organic nutrients from associated soil fungi. Despite involving radical changes in life history traits and ecological requirements, the transition from autotrophy Edited by: Susann Wicke, to mycoheterotrophy has occurred independently in many major lineages of land Humboldt University of Berlin, plants, most frequently in Orchidaceae. Yet the molecular mechanisms underlying this Germany shift are still poorly understood. A comparison of the transcriptomes of Epipogium Reviewed by: Maria D. Logacheva, aphyllum and Neottia nidus-avis, two completely mycoheterotrophic orchids, to other Skolkovo Institute of Science autotrophic and mycoheterotrophic orchids showed the unexpected retention of several and Technology, Russia genes associated with photosynthetic activities. In addition to these selected retentions, Sean W. Graham, University of British Columbia, the analysis of their expression profiles showed that many orthologs had inverted Canada underground/aboveground expression ratios compared to autotrophic species. Fatty Craig Barrett, West Virginia University, United States acid and amino acid biosynthesis as well as primary cell wall metabolism were among *Correspondence: the pathways most impacted by this expression reprogramming. -

Typification of Infrageneric Taxa in Dendrobium(Orchidaceae)
Typification of infrageneric taxa in Dendrobium (Orchidaceae) André Schuiteman Royal Botanic Gardens, Kew, Richmond, Surrey, TW9 3AB, United Kingdom; e-mail: [email protected] Introduction Abstract The nomenclature of infrageneric taxa in Orchidaceae on occasion In order to stabilise the nomenclature borders on the chaotic. In the past, names were often proposed in a of infrageneric taxa in Dendrobium, type species are chosen for those highly informal fashion, with little concern for typification, priority, taxa for which this has not been precise circumscription, or even ranking. Such names were frequently done previously. In all, 35 names are applied to sets of species which had little in common beyond one or typified, including one genus name a few key characters. Those who used these names rarely cited their (Onychium), and assigned to sections authors, while the circumscription of the taxa often varied considerably of Dendrobium as recognised in Genera Orchidacearum. from one botanist to the next. It was not uncommon that earlier names were deliberately disregarded, to be replaced with new names that Keywords: Dendrobium, infrageneric taxa, nomenclature, taxonomy more or less covered the same groups. As a result, the nomenclature and systematics of many infrageneric taxa were, and sometimes still are, Muelleria 30(1): 3–7 (2012) extremely confused. In Dendrobium, one of the largest orchid genera with some 1500 species (Pridgeon et al., in prep.), well over 200 subgenera, sections and subsections have been proposed. Examples of all the problems just mentioned can be found in abundance in this gargantuan taxon. Fortunately, many of these problems can be solved rather easily by carefully typifying the taxa involved. -
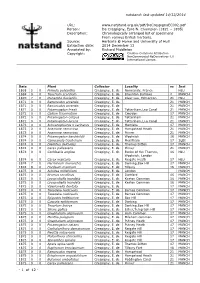
Date Plant Collector Locality Vc Inst 1868 5 0 Primula Polyantha Crespigny, E
natstand: last updated 14/12/2014 URL: www.natstand.org.uk/pdf/DeCrespignyEC002.pdf Person: De Crespigny, Eyre N. Champion (1821 – 1895) Description: Chronologically arranged list of specimens From various British herbaris. Source: Herbaria @ Home and University of Hull Extraction date: 2014 December 13 Annotated by: Richard Middleton Copyright: Creative Commons Attribution- NonCommercial-NoDerivatives 4.0 International License. Date Plant Collector Locality vc Inst 1868 5 0 Primula polyantha Crespigny, E. de Normandy, France HLU 1869 0 0 Teucrium scordium Crespigny, E. de Braunton Burrows 4 MANCH 1870 7 0 Oenanthe fluviatilis Crespigny, E. de River Lee, Edmonton 21 HLU 1871 0 0 Ranunculus arvensis Crespigny, E. de 21 MANCH 1871 0 0 Ranunculus arvensis Crespigny, E. de 21 MANCH 1871 0 0 Potamogeton friesii Crespigny, E. de Tottenham,Lea Canal 21 MANCH 1872 0 0 Galium tricornutum Crespigny, E. de Croydon 17 MANCH 1872 0 0 Potamogeton crispus Crespigny, E. de Tottenham 21 MANCH 1872 0 0 Potamogeton lucens Crespigny, E. de Tottenham,Lea Canal 21 MANCH 1873 0 0 Schoenoplectus x carinatus Crespigny, E. de Mortlake 17 MANCH 1873 0 0 Anemone nemorosa Crespigny, E. de Hampstead Heath 21 MANCH 1873 0 0 Anemone nemorosa Crespigny, E. de Pinner 21 MANCH 1874 0 0 Potamogeton berchtoldii Crespigny, E. de Woolwich 16 MANCH 1874 0 0 Campanula trachelium Crespigny, E. de Merstham 17 SLBI 1874 0 0 Dianthus deltoides Crespigny, E. de Thames Ditton 17 MANCH 1874 0 0 Carex pallescens Crespigny, E. de Pinner 21 MANCH 1874 0 0 Cochlearia anglica Crespigny, E. de Banks of the Thames, 16 HLU Woolwich, London 1874 6 0 Carex vesicaria Crespigny, E. -

Phylogenetic Placement of the Enigmatic Orchid Genera Thaia and Tangtsinia: Evidence from Molecular and Morphological Characters
TAXON 61 (1) • February 2012: 45–54 Xiang & al. • Phylogenetic placement of Thaia and Tangtsinia Phylogenetic placement of the enigmatic orchid genera Thaia and Tangtsinia: Evidence from molecular and morphological characters Xiao-Guo Xiang,1 De-Zhu Li,2 Wei-Tao Jin,1 Hai-Lang Zhou,1 Jian-Wu Li3 & Xiao-Hua Jin1 1 Herbarium & State Key Laboratory of Systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, P.R. China 2 Key Laboratory of Biodiversity and Biogeography, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan 650204, P.R. China 3 Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Menglun Township, Mengla County, Yunnan province 666303, P.R. China Author for correspondence: Xiao-Hua Jin, [email protected] Abstract The phylogenetic position of two enigmatic Asian orchid genera, Thaia and Tangtsinia, were inferred from molecular data and morphological evidence. An analysis of combined plastid data (rbcL + matK + psaB) using Bayesian and parsimony methods revealed that Thaia is a sister group to the higher epidendroids, and tribe Neottieae is polyphyletic unless Thaia is removed. Morphological evidence, such as plicate leaves and corms, the structure of the gynostemium and the micromorphol- ogy of pollinia, also indicates that Thaia should be excluded from Neottieae. Thaieae, a new tribe, is therefore tentatively established. Using Bayesian and parsimony methods, analyses of combined plastid and nuclear datasets (rbcL, matK, psaB, trnL-F, ITS, Xdh) confirmed that the monotypic genus Tangtsinia was nested within and is synonymous with the genus Cepha- lanthera, in which an apical stigma has evolved independently at least twice. -

57. CEPHALANTHERA Richard, De Orchid. Eur. 21, 29, 38. 1817. 头蕊兰属 Tou Rui Lan Shu Chen Xinqi (陈心启 Chen Sing-Chi); Stephan W
Flora of China 25: 174–177. 2009. 57. CEPHALANTHERA Richard, De Orchid. Eur. 21, 29, 38. 1817. 头蕊兰属 tou rui lan shu Chen Xinqi (陈心启 Chen Sing-chi); Stephan W. Gale, Phillip J. Cribb Callithronum Ehrhart; Dorycheile Reichenbach; Eburophyton A. Heller; Xiphophyllum Ehrhart. Herbs, terrestrial, autotrophic or holomycotrophic. Rhizome creeping, cylindric, slender; roots fasciculate, filiform, fleshy, usually numerous though few in holomycotrophic species. Stem erect, unbranched, leafy, with 1 to a few subcymbiform or cylindric basal sheaths. Leaves alternate, plicate, sessile, directly sheathing stem at base, reduced to membranous sheaths in holomycotrophic species. Inflorescence terminal, racemose, many or few flowered, rarely 1-flowered; proximal floral bracts foliaceous and usually longer than flowers, distal ones much shorter. Flowers resupinate, suberect, weakly spreading and campanulate, or rarely widely spreading, white, pink, or yellow; ovary slightly twisted, glabrous. Sepals free, similar to each other, subequal. Petals slightly shorter than sepals, ± connivent with sepals; lip adnate to base of column, 2-partite or rarely simple and not distinct from petals in peloric forms; hypochile with erect lateral lobes embracing column, saccate or with a short spur at base; epichile spreading, ovate-elliptic, apex obtuse or acute; disk with 3–7 longitudinal lamellae, or unornamented in peloric forms. Column erect, usually with 2 narrow lateral wings; anther erect, hinged, 2-locular; pollinia 2, each 2-partite, granular-farinaceous, lacking caudicles and viscidia; stigma concave, rounded; rostellum inconspicuous or absent. Capsule erect. About 15 species: mainly in Europe, N Africa, and E Asia, but also in the Himalayas, SE Asia, and extending to the west coast of North America; nine species (four endemic) in China. -

Dispersion of Vascular Plant in Mt. Huiyangsan, Korea
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Journal of Korean Nature Vol. 3, No. 1 1-10, 2010 Dispersion of Vascular Plant in Mt. Huiyangsan, Korea Hyun-Tak Shin1, Sung-Tae Yoo2, Byung-Do Kim2, and Myung-Hoon YI3* 1Gyeongsangnam-do Forest Environment Research Institute, Jinju 660-871, Korea 2Daegu Arboretum 284 Daegok-Dong Dalse-Gu Daegu 704-310, Korea 3Department of Landscape Architecture, Graduate School, Yeungnam University, Gyeongsan 712-749, Korea Abstract: We surveyed that vascular plants can be classified into 90 families and 240 genus, 336 species, 69 variants, 22 forms, 3 subspecies, total 430 taxa. Dicotyledon plant is 80.9%, monocotyledon plant is 9.8%, Pteridophyta is 8.1%, Gymnosermae is 1.2% among the whole plant family. Rare and endangered plants are Crypsinus hastatus, Lilium distichum, Viola albida, Rhododendron micranthum, totalling four species. Endemic plants are Carex okamotoi, Salix koriyanagi for. koriyanagi, Clematis trichotoma, Thalictrum actaefolium var. brevistylum, Galium trachyspermum, Asperula lasiantha, Weigela subsessilis, Adenophora verticillata var. hirsuta, Aster koraiensis, Cirsium chanroenicum and Saussurea seoulensis total 11 taxa. Specialized plants are 20 classification for I class, 7 classifications for the II class, 7 classifications for the III class, 2 classification for the IV class, and 1 classification for the V class, total 84 taxa. Naturalized plants specified in this study are 10 types but Naturalization rate is not high compared to the area of BaekDu-DaeGan. This survey area is focused on the center of BaekDu- DaeGan, and it has been affected by excessive investigations and this area has been preserved as Buddhist temples' woods. -

Click to View .Pdf of This Issue
JJoouurrnnaall of the HHAARRDDYY OORRCCHHIIDD SSOOCCIIEETTYY Vol. 6 No. 2 (52) April 2009 JOURNAL of the HARDY ORCHID SOCIETY Vol. 6 No. 2 (52) April 2009 The Hardy Orchid Society Our aim is to promote interest in the study of Native European Orchids and those from similar temperate climates throughout the world. We cover such varied aspects as field study, cultivation and propagation, photography, taxonomy and systematics, and practical conservation. We welcome articles relating to any of these subjects, which will be considered for publication by the editorial committee. Please send your submissions to the Editor, and please structure your text according to the “Advice to Authors” (see website, January 2004 Journal, Members’ Handbook or contact the Editor). Views expressed in journal articles are those of their author(s) and may not reflect those of HOS. The Hardy Orchid Society Committee President: Prof. Richard Bateman, Jodrell Laboratory, Royal Botanic Gardens Kew, Richmond, Surrey, TW9 3DS Chairman & Field Meeting Co-ordinator: David Hughes, Linmoor Cottage, Highwood, Ringwood, Hants., BH24 3LE [email protected] Vice-Chairman: Celia Wright, The Windmill, Vennington, Westbury, Shrewsbury, Shropshire, SY5 9RG [email protected] Secretary (Acting): Alan Leck, 61 Fraser Close, Deeping St. James, Peterborough, PE6 8QL [email protected] Treasurer: Iain Wright, The Windmill, Vennington, Westbury, Shrewsbury, Shropshire, SY5 9RG [email protected] Membership Secretary: Celia Wright, The Windmill, Vennington, Westbury, -

The Real Ponerorchis Nana (King & Pantling) Soó Resurrected
Pleione 10(2): 279 - 282. 2016. ISSN: 0973-9467 © East Himalayan Society for Spermatophyte Taxonomy The real Ponerorchis nana (King & Pantling) Soó resurrected Magnus Lidén1 and Alister Adhikari2 1Uppsala university, EBC: Systematic Biology. Norbyvägen 18D, 75236 Uppsala, Sweden. E-mail: [email protected]. 2 Dr. Graham’s Homes, Kalimpong 734301, West Bengal. E-mail: [email protected]. [Received 01.11.2016; Revised & accepted 04.11.2016; Published 31.12.2016] Abstract We report a find of the rare orchid Ponerorchis nana (King & Pantling) Soó (Orchidaceae) from Lachung, Sikkim, and compare it with the very different species P. chusua with which it has previously been associated. Ponerorchis nana is currently known from East Sikkim Eastwards to Central Arunachal Pradesh, and grows on moss-covered cliffs and tree trunks. It seems closely related to Amitostigma pathakianum. Key words: Ponerorchis nana, Identity, Reestablished species Ponerorchis nana (King & Pantling) Soó (Orchidaceae) is a much misunderstood taxon. In Flora of Bhutan (Pearce & Cribb 2002) and on most websites (see references: web-resources) P. nana is said to be either very similar to or synonymous with P. chusua, and the epithet has been used for both narrow-leaved and broad-leaved small individuals of P. Chusua (e.g. Adhikari 2008). The root of the confusion started long lack when King & Pantling (1898) originally described P. nana as a variety of P. chusua and even hinted at intermediates. However, Ponerorchis nana (Figures 1, 2) is very different from P. chusua (Figure 3), in morphology as well as in ecology, and no intermediates are known. Pantling’s original drawing (King & Pantling 1898) shows most of its distinctive features: small and delicate growth; a single linear arcuate channeled leaf with shortly clasping base; 1- to 2-flowered (very rarely 3-flowered) inflorescence; flowers less than half the size of those of P.