Understanding and Explaining Viral Vector COVID-19 Vaccines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mobile Genetic Elements in Streptococci
Curr. Issues Mol. Biol. (2019) 32: 123-166. DOI: https://dx.doi.org/10.21775/cimb.032.123 Mobile Genetic Elements in Streptococci Miao Lu#, Tao Gong#, Anqi Zhang, Boyu Tang, Jiamin Chen, Zhong Zhang, Yuqing Li*, Xuedong Zhou* State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu, PR China. #Miao Lu and Tao Gong contributed equally to this work. *Address correspondence to: [email protected], [email protected] Abstract Streptococci are a group of Gram-positive bacteria belonging to the family Streptococcaceae, which are responsible of multiple diseases. Some of these species can cause invasive infection that may result in life-threatening illness. Moreover, antibiotic-resistant bacteria are considerably increasing, thus imposing a global consideration. One of the main causes of this resistance is the horizontal gene transfer (HGT), associated to gene transfer agents including transposons, integrons, plasmids and bacteriophages. These agents, which are called mobile genetic elements (MGEs), encode proteins able to mediate DNA movements. This review briefly describes MGEs in streptococci, focusing on their structure and properties related to HGT and antibiotic resistance. caister.com/cimb 123 Curr. Issues Mol. Biol. (2019) Vol. 32 Mobile Genetic Elements Lu et al Introduction Streptococci are a group of Gram-positive bacteria widely distributed across human and animals. Unlike the Staphylococcus species, streptococci are catalase negative and are subclassified into the three subspecies alpha, beta and gamma according to the partial, complete or absent hemolysis induced, respectively. The beta hemolytic streptococci species are further classified by the cell wall carbohydrate composition (Lancefield, 1933) and according to human diseases in Lancefield groups A, B, C and G. -

Ifs Recommendations for Covid 19 Vaccination Before Art
16 – 17 JULY 2020 PRAGATI MAIDAN - DELHI INDIAN FERTILITY SOCIETY IFS RECOMMENDATIONS FOR COVID 19 VACCINATION BEFORE ART IFS SECRETARIAT +91 11 40018184 +91 9899308083 indianferlitysociety [email protected] www.indianferlitysociety.org indianferlitysociety 302, 3rd Floor, Kailash Building, ifsdelhi 26, Kasturba Gandhi Marg, C.P. New Delhi - 110001 Cosmo Tech is the Most Important Trade show for the Suppliers to EXPAND Strengthen Increase Multiply Profits New Markets Pan New Market Customer Base India & World Customer Soaring Boost Network Brand Sales Brand Recall Value with the Industry Visibility ORGANIZED BY CALL EMAIL +91 9971811937 [email protected] +91 9999302797 [email protected] +91 9811141938 [email protected] W W W .C O SMO TEC HEXPO IND IA .C O M Dr. Sudha Prasad Dr. Neena Malhotra President Secretary General Indian Fertility Society Indian Fertility Society Director, Matritava Advanced IVF & Professor, ART Centre, Department of Training Cenre, Vasant Vihar, New Delhi Obstetrics and Gynaecology, AIIMS, New Delhi Dr. Sonia Malik Dr. Kuldeep Jain Past President Indian Fertility Society, Past President Indian Fertility Society, Director & Nova Southend Fertility & Director, KJIVF New Delhi IVF Delhi NCR Dr. KU Kunjumoideen Dr. A K Pandey Joint Secretary Indian Fertility Society, Dean Academics &. Co Ordinator Director ARMC IVF Calicut. Molecular Lab. ESI Medical college Faridabad Dr. Charu Jandial Dr. Sumita Aggarwal Member IFS, Consultant, Member IFS, Fellow, Nova Southend Fertility & IVF Delhi NCR Nova Southend Fertility & IVF Delhi NCR Introduction The coronavirus pandemic has wreaked havoc on life In these trying mes, as sociees are gradually trying to and healthcare globally. According to WHO database as return to a state of normalcy, it is also important to on 7th June 2021, there have been 173 million consider sexual and reproducve health of people. -

Gutless Adenovirus: Last-Generation Adenovirus for Gene Therapy
Gene Therapy (2005) 12, S18–S27 & 2005 Nature Publishing Group All rights reserved 0969-7128/05 $30.00 www.nature.com/gt CONFERENCE PAPER Gutless adenovirus: last-generation adenovirus for gene therapy R Alba1, A Bosch1 and M Chillon1,2 1Gene Therapy Laboratory, Department of Biochemistry and Molecular Biology, Center of Animal Biotechnology and Gene Therapy (CBATEG), Universitat Auto`noma de Barcelona, Bellaterra, Spain; and 2Institut Catala` de Recerca i Estudis Avanc¸ats (ICREA), Barcelona, Spain Last-generation adenovirus vectors, also called helper-depen- viral coding regions, gutless vectors require viral proteins dent or gutless adenovirus, are very attractive for gene therapy supplied in trans by a helper virus. To remove contamination because the associated in vivo immune response is highly by a helper virus from the final preparation, different systems reduced compared to first- and second-generation adenovirus based on the excision of the helper-packaging signal have vectors, while maintaining high transduction efficiency and been generated. Among them, Cre-loxP system is mostly tropism. Nowadays, gutless adenovirus is administered in used, although contamination levels still are 0.1–1% too high different organs, such as the liver, muscle or the central to be used in clinical trials. Recently developed strategies to nervous system achieving high-level and long-term transgene avoid/reduce helper contamination were reviewed. expression in rodents and primates. However, as devoid of all Gene Therapy (2005) 12, S18–S27. doi:10.1038/sj.gt.3302612 Keywords: adenovirus; gutless; helper-dependent vectors; in vivo gene therapy Introduction clinical for more information). Nowadays, adenovirus vectors are applied to treat cancer, monogenic disorders, Gene therapy for most genetic diseases requires expres- vascular diseases and others complications. -

Review of COVID-19 Vaccine Subtypes, Efficacy and Geographical Distributions Andre Ian Francis ,1 Saudah Ghany,1 Tia Gilkes,1 Srikanth Umakanthan2
Review Postgrad Med J: first published as 10.1136/postgradmedj-2021-140654 on 6 August 2021. Downloaded from Review of COVID-19 vaccine subtypes, efficacy and geographical distributions Andre Ian Francis ,1 Saudah Ghany,1 Tia Gilkes,1 Srikanth Umakanthan2 1Department of Clinical Medical ABSTRACT 2021, the Ad26.COV2.S, developed by Janssen Sciences, The Faculty of Medical As of 1 May 2021, there have been 152 661 445 (Johnson & Johnson) and Moderna on 30 April.4 Sciences, The University of the COVAX, coordinated by WHO, Gavi: The West Indies, St. Augustine, Covid-19 cases with 3 202 256 deaths globally. Trinidad and Tobago This pandemic led to the race to discover a vaccine Vaccine Alliance, the Coalition for Epidemic 2Pathology unit, Department to achieve herd immunity and curtail the damaging Preparedness Innovations (CEPI), acts as a of Paraclinical Sciences, The effects of Covid-19. This study aims to discuss the most programme that supports the development of University Of The West Indies, St. COVID-19 vaccine candidates and negotiates their Augustine, Trinidad and Tobago recent WHO-approved Covid-19 vaccine subtypes, their status and geographical scheduled updates as of 4 pricing to ensure low- and- middle- income countries have a fair shot at receiving vaccines.5 Correspondence to May 2021. The keywords “Covid-19, Vaccines, Pfizer, Mr. Andre Ian Francis, The BNT162b2, AstraZeneca, AZD1222, Moderna, mRNA- This article aims at discussing the most recent University of the West Indies, St. 1273, Janssen, Ad26.COV2.S” were typed into PubMed. WHO- approved COVID-19 vaccine subtypes, their Augustine, Trinidad and Tobago; Thirty Two relevant PubMed articles were included in status and geographical scheduled updates as of 4 andre. -

Viral Vectors and Biological Safety
Viral Vectors and Biological Safety Viral vectors are often designed so that they can enter human cells and deliver genes of interest. Viral vectors are usually replication-deficient – genes necessary for replication of the virus are removed from the vector and supplied separately through plasmids, helper virus, or packaging cell lines. There are several biosafety concerns that arise with the use of viral vectors including: 1) Tropism (host range) – viral vectors that can enter (infect) human cells are often used. 2) Replication-deficient viral vectors can gain back the deleted genes required for replication (become replication-competent) through recombination – referred to as replication-competent virus (RCV) breakthroughs. 3) Genes may be expressed in tissues and/or organisms where they are normally not expressed. In the case of some genes such as oncogenes, this could have far-reaching negative consequences. When evaluating safety for use of viral vectors, a number of factors need to be considered including: Risk Group (RG) of the organism; tropism (organism and tissue); route of transmission; whether the virus integrates into the host genome; and the specific gene(s) being introduced. Please contact the Office of Biological Safety (OBS) for more information on physical barriers and safety practices to use with specific viral vectors. This article concentrates on biological barriers that can be employed to improve safety when using viral vectors. Viral vectors frequently used are: • Retrovirus/lentivirus • Adenovirus • Adeno-associated virus (AAV) • Poxvirus • Herpes virus • Alphavirus • Baculovirus Amphotropic murine leukemia virus (MLV) – also called Moloney murine leukemia virus (MMLV) – and adenovirus are common viral vectors used to introduce genes into human cells. -
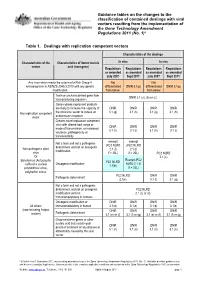
Guidance Tables on the Changes To
Guidance tables on the changes to the classification of contained dealings with viral vectors resulting from the implementation of the Gene Technology Amendment Regulations 2011 (No. 1)* Table 1. Dealings with replication competent vectors Characteristics of the dealings Characteristics of the Characteristics of donor nucleic In vitro In vivo vector acid (transgene) Regulations Regulations Regulations Regulations as amended as amended as amended as amended July 2007 Sept 2011* July 2007 Sept 2011* Any virus which meets the criteria of a Risk Group 4 Not Not microorganism in AS/NZS 2243.3:2010 with any genetic differentiated DNIR 3.1(p) differentiated DNIR 3.1(p) modification from below from below Toxin or uncharacterised gene from DNIR 3.1 (a), (b) or (c) toxin producing organism Genes whose expressed products are likely to increase the capacity of DNIR DNIR DNIR DNIR Any replication competent the virus/viral vector to induce an 3.1 (g) 3.1 (h) 3.1 (g) 3.1 (h) vector autoimmune response Creates novel replication competent virus with altered host range or DNIR DNIR DNIR DNIR mode of transmission, or increased 3.1 (h) 3.1 (i) 3.1 (h) 3.1 (i) virulence, pathogenicity or transmissibility exempt exempt Not a toxin and not a pathogenic (PC2 NLRD (PC2 NLRD determinant and not an oncogenic Non-pathogenic plant 2.1 (f) 2.1 (f) modification virus if > 25L) if > 25L) PC2 NLRD Or 2.1 (c) Exempt (PC2 Baculovirus (Autographa PC1 NLRD Oncogenic modification NLRD 2.1 (f) californica nuclear 1.1(b) polyhedrosis virus), if > 25L) polyhedrin minus PC2 NLRD -

Sars-Cov-2 Infection in Healthcare Workers and Their Household Contacts at the University of North Carolina Medical Center
VECTOR OR VICTIM: SARS-COV-2 INFECTION IN HEALTHCARE WORKERS AND THEIR HOUSEHOLD CONTACTS AT THE UNIVERSITY OF NORTH CAROLINA MEDICAL CENTER Team Co-PIs: Ross Boyce, MD (SOM) and Allison Aiello, PhD (SPH) Coinvestigators: David Richardson, PhD (SPH), David Weber, MD (SOM), Emily Sickbert-Bennett, PhD, MS (SOM), Erica Pettigrew Md, JD, MPH (SOM), Raquel Reyes, MD (Hospital Medicine), Naseem Alavian, MD (Hospital Medicine), Jon Juliano, MD, MPH (SOM) Emily Ciccone MD,MHS (SOM), Billy Fischer, MD (SOM) and Subha Sellers, MD,MSc (SOM) The COVID-19 pandemic now accounts for more than 1.1 million confirmed infections and nearly than 70,000 deaths in the United States (US), along with unprecedented disruption to social networks and economic systems. The causative agent, the SARS-CoV-2 coronavirus, is primarily spread from person-to-person through the inhalation or direct contact with aerosolized droplets. Frontline healthcare workers (HCW) are at increased risk of infection due to frequent exposure to and close contact with infected patients and contaminated surfaces. Shortages of critical personal protective equipment (PPE) may further exacerbate this risk. A review of the epidemiological data from the site of the initial COVID-19 outbreak in Wuhan, China showed that 63% of HCWs were infected with SARS- CoV-2, many of who developed severe disease. While data from US facilities is still emerging, an analysis of case reports submitted to the Centers for Disease Control and Prevention (CDC) found nearly 10,000 cases of COVID-19 among HCWs. Infected HCWs can also contribute to disease transmission, both in the hospital setting and in the community. -

Climate Change and Vector-Borne/Zoonotic Diseases
Climate Change and Vector-Borne/Zoonotic Diseases Kenneth L. Gage Division of Vector-Borne Infectious Diseases National Center for Zoonotic, Vector-Borne and Enteric Diseases Centers for Disease Control and Prevention Vector Disease agents Threshold for Effects of Climatic Factors Biological Activity on Hosts and Vectors* Anopheles Plasmodium sp. 8-10o C mosquitoes • Growth, development and reproduction Triatomine Trypanosoma 20o C – Q10 effects (approximate doubling of bugs cruzi (2-6o C for survival) metabolic rates in poikliothermic organisms with 10oC rise in temperatures) Aedes Dengue virus 6-10o C – Rate of reproduction/Number of mosquitoes generations per season – Example: Anopheles gambiae gonotrophic Ixodes ticks Borrelia 5-8o C cycles significantly shorter in open treeless burdgdorferi, sites (warmer) than forested sites (cooler) Anaplasma • Activity patterns phagocytophilum, – Feeding Babesia microti – Host seeking o – Mate seeking etc. Bulinus and Schistosoma sp. 5 C o • Availability of breeding sites other snails (25+2 C optimal) • Survival – Severe weather events Source: Patz and Olson 2006 – Tolerance limits for vectors and hosts – Food or water availability – Freezing or heat stress Effect of Temperature on Oxygen Consumption * See Gubler et al. 2001 for citations and additional examples Climate Effects on Hosts and Vectors - Distribution and Abundance - • “Weather school” (Andrewartha and Birch 1954) – Changing conditions make areas more or less suitable for survival and reproduction, which affects abundance of different species – Changing conditions often related to climatic variables (temperature, precipitation, humidity, etc.) – Most extreme effects seen for insects and other arthropods • Host or vector populations can increase during favorable conditions and later crash as conditions deteriorate • Many examples with epidemiologic significance – Mosquito vectors • Rift valley fever (arbovirus)(Linthicum et al. -

Different Types of COVID-19 Vaccines
Pfizer-BioNTech Help stop the pandemic Type of vaccine: Messenger RNA, or mRNA, a genetic Different Types material that tells your body how to make proteins that by getting vaccinated triggers an immune response inside our bodies of COVID-19 Effectiveness: 95% based on clinical trials Even if you are undocumented Common side effects: Pain and/or swelling in the arm and/or don’t have insurance, you and tiredness, headache, muscle pain, chills, fever, or can get the vaccine—for free. Vaccines: nausea in the body that may last two days Recommended Ages: 16 and older (currently testing the vaccine in kids ages 12-15) Understanding How Dosage: Two shots, 21 days apart They Work Visit VaccinateALL58.com Moderna for the newest information about when and where the vaccine Type of vaccine: Messenger RNA, or mRNA, a genetic will be available to you. material that tells your body how to make proteins that triggers an immune response inside our bodies Sign up at myturn.ca.gov or Effectiveness: 94.1% based on clinical trials call 1-833-422-4255 to find out Common side effects: Pain and/or swelling in the arm if it’s your turn to get vaccinated and and tiredness, headache, muscle pain, chills, fever, or schedule vaccination appointments. nausea in the body that may last two days Recommended Ages: 18 years and older (currently testing the vaccine in kids ages 12-17) Dosage: Two shots, 28 days apart Johnson & Johnson Follow us on social media for more COVID-19 tips and information. Type of vaccine: A viral vector, it uses a harmless version of a different -

A National Public Health Framework for the Prevention and Control of Vector-Borne Diseases in Humans
A National Public Health Framework for the Prevention and Control of Vector-Borne Diseases in Humans WWW.CDC.GOV/VECTOR 1 Amblyomma maculatum Publication and Copyright Information Centers for Disease Control and Prevention A National Public Health Framework for the Prevention and Control of Vector-Borne Diseases in Humans Atlanta, Georgia: September 2020 www.cdc.gov/vector Media inquiries: 404-639-3286 (9:00 am–6:00 pm ET); [email protected] Acknowledgement: Layout and graphics provided by CDC’s Creative Services. Cover Clockwise from top right: • Blacklegged tick (Ixodes scapularis), James Gathany photographer • Aedes aegypti mosquito, James Gathany photographer • Illustration of the United States • Oriental rat flea Xenopsylla( cheopsis), James Gathany photographer Accessible Version: www.cdc.gov/ncezid/dvbd/framework.html 2 A NATIONAL PUBLIC HEALTH FRAMEWORK FOR THE PREVENTION AND CONTROL OF VECTOR-BORNE DISEASES IN HUMANS Introduction and Scope Our nation’s ability to defend against the present the U.S. population from these diseases, five federal and future threat of vector-borne diseases relies on a departments and the Environmental Protection Agency comprehensive national system that is able to detect, contributed to developing a national framework for vector- prevent, and respond to these threats. A concerted borne disease prevention and control. These federal and sustained effort is needed to address significant partners represent the primary federal departments and challenges and reverse the upward trends in illness, agencies engaged -

Chapter 2 Disease and Disease Transmission
DISEASE AND DISEASE TRANSMISSION Chapter 2 Disease and disease transmission An enormous variety of organisms exist, including some which can survive and even develop in the body of people or animals. If the organism can cause infection, it is an infectious agent. In this manual infectious agents which cause infection and illness are called pathogens. Diseases caused by pathogens, or the toxins they produce, are communicable or infectious diseases (45). In this manual these will be called disease and infection. This chapter presents the transmission cycle of disease with its different elements, and categorises the different infections related to WES. 2.1 Introduction to the transmission cycle of disease To be able to persist or live on, pathogens must be able to leave an infected host, survive transmission in the environment, enter a susceptible person or animal, and develop and/or multiply in the newly infected host. The transmission of pathogens from current to future host follows a repeating cycle. This cycle can be simple, with a direct transmission from current to future host, or complex, where transmission occurs through (multiple) intermediate hosts or vectors. This cycle is called the transmission cycle of disease, or transmission cycle. The transmission cycle has different elements: The pathogen: the organism causing the infection The host: the infected person or animal ‘carrying’ the pathogen The exit: the method the pathogen uses to leave the body of the host Transmission: how the pathogen is transferred from host to susceptible person or animal, which can include developmental stages in the environment, in intermediate hosts, or in vectors 7 CONTROLLING AND PREVENTING DISEASE The environment: the environment in which transmission of the pathogen takes place. -

Data–Model Fusion to Better Understand Emerging Pathogens and Improve Infectious Disease Forecasting
July 2011 DATA ASSIMILATION FOR ECOLOGICAL FORECASTING Ecological Applications, 21(5), 2011, pp. 1443–1460 Ó 2011 by the Ecological Society of America Data–model fusion to better understand emerging pathogens and improve infectious disease forecasting 1,5 2 3 4 1 SHANNON L. LADEAU, GREGORY E. GLASS, N. THOMPSON HOBBS, ANDREW LATIMER, AND RICHARD S. OSTFELD 1Cary Institute of Ecosystem Studies, Millbrook, New York 12545 USA 2Johns Hopkins School of Public Health, Department of Molecular Microbiology and Immunology, Baltimore, Maryland 21205 USA 3Colorado State University, Natural Resource Ecology Laboratory and Graduate Degree Program in Ecology, Fort Collins, Colorado 80524 USA 4University of California, Department of Plant Sciences, Davis, California 95616 USA Abstract. Ecologists worldwide are challenged to contribute solutions to urgent and pressing environmental problems by forecasting how populations, communities, and ecosystems will respond to global change. Rising to this challenge requires organizing ecological information derived from diverse sources and formally assimilating data with models of ecological processes. The study of infectious disease has depended on strategies for integrating patterns of observed disease incidence with mechanistic process models since John Snow first mapped cholera cases around a London water pump in 1854. Still, zoonotic and vector-borne diseases increasingly affect human populations, and methods used to successfully characterize directly transmitted diseases are often insufficient. We use four case studies to demonstrate that advances in disease forecasting require better understanding of zoonotic host and vector populations, as well of the dynamics that facilitate pathogen amplification and disease spillover into humans. In each case study, this goal is complicated by limited data, spatiotemporal variability in pathogen transmission and impact, and often, insufficient biological understanding.