Twiv 206 Transcript
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mobile Genetic Elements in Streptococci
Curr. Issues Mol. Biol. (2019) 32: 123-166. DOI: https://dx.doi.org/10.21775/cimb.032.123 Mobile Genetic Elements in Streptococci Miao Lu#, Tao Gong#, Anqi Zhang, Boyu Tang, Jiamin Chen, Zhong Zhang, Yuqing Li*, Xuedong Zhou* State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu, PR China. #Miao Lu and Tao Gong contributed equally to this work. *Address correspondence to: [email protected], [email protected] Abstract Streptococci are a group of Gram-positive bacteria belonging to the family Streptococcaceae, which are responsible of multiple diseases. Some of these species can cause invasive infection that may result in life-threatening illness. Moreover, antibiotic-resistant bacteria are considerably increasing, thus imposing a global consideration. One of the main causes of this resistance is the horizontal gene transfer (HGT), associated to gene transfer agents including transposons, integrons, plasmids and bacteriophages. These agents, which are called mobile genetic elements (MGEs), encode proteins able to mediate DNA movements. This review briefly describes MGEs in streptococci, focusing on their structure and properties related to HGT and antibiotic resistance. caister.com/cimb 123 Curr. Issues Mol. Biol. (2019) Vol. 32 Mobile Genetic Elements Lu et al Introduction Streptococci are a group of Gram-positive bacteria widely distributed across human and animals. Unlike the Staphylococcus species, streptococci are catalase negative and are subclassified into the three subspecies alpha, beta and gamma according to the partial, complete or absent hemolysis induced, respectively. The beta hemolytic streptococci species are further classified by the cell wall carbohydrate composition (Lancefield, 1933) and according to human diseases in Lancefield groups A, B, C and G. -

Chapitre Quatre La Spécificité D'hôtes Des Virophages Sputnik
AIX-MARSEILLE UNIVERSITE FACULTE DE MEDECINE DE MARSEILLE ECOLE DOCTORALE DES SCIENCES DE LA VIE ET DE LA SANTE THESE DE DOCTORAT Présentée par Morgan GAÏA Né le 24 Octobre 1987 à Aubagne, France Pour obtenir le grade de DOCTEUR de l’UNIVERSITE AIX -MARSEILLE SPECIALITE : Pathologie Humaine, Maladies Infectieuses Les virophages de Mimiviridae The Mimiviridae virophages Présentée et publiquement soutenue devant la FACULTE DE MEDECINE de MARSEILLE le 10 décembre 2013 Membres du jury de la thèse : Pr. Bernard La Scola Directeur de thèse Pr. Jean -Marc Rolain Président du jury Pr. Bruno Pozzetto Rapporteur Dr. Hervé Lecoq Rapporteur Faculté de Médecine, 13385 Marseille Cedex 05, France URMITE, UM63, CNRS 7278, IRD 198, Inserm 1095 Directeur : Pr. Didier RAOULT Avant-propos Le format de présentation de cette thèse correspond à une recommandation de la spécialité Maladies Infectieuses et Microbiologie, à l’intérieur du Master des Sciences de la Vie et de la Santé qui dépend de l’Ecole Doctorale des Sciences de la Vie de Marseille. Le candidat est amené à respecter des règles qui lui sont imposées et qui comportent un format de thèse utilisé dans le Nord de l’Europe permettant un meilleur rangement que les thèses traditionnelles. Par ailleurs, la partie introduction et bibliographie est remplacée par une revue envoyée dans un journal afin de permettre une évaluation extérieure de la qualité de la revue et de permettre à l’étudiant de commencer le plus tôt possible une bibliographie exhaustive sur le domaine de cette thèse. Par ailleurs, la thèse est présentée sur article publié, accepté ou soumis associé d’un bref commentaire donnant le sens général du travail. -

Gutless Adenovirus: Last-Generation Adenovirus for Gene Therapy
Gene Therapy (2005) 12, S18–S27 & 2005 Nature Publishing Group All rights reserved 0969-7128/05 $30.00 www.nature.com/gt CONFERENCE PAPER Gutless adenovirus: last-generation adenovirus for gene therapy R Alba1, A Bosch1 and M Chillon1,2 1Gene Therapy Laboratory, Department of Biochemistry and Molecular Biology, Center of Animal Biotechnology and Gene Therapy (CBATEG), Universitat Auto`noma de Barcelona, Bellaterra, Spain; and 2Institut Catala` de Recerca i Estudis Avanc¸ats (ICREA), Barcelona, Spain Last-generation adenovirus vectors, also called helper-depen- viral coding regions, gutless vectors require viral proteins dent or gutless adenovirus, are very attractive for gene therapy supplied in trans by a helper virus. To remove contamination because the associated in vivo immune response is highly by a helper virus from the final preparation, different systems reduced compared to first- and second-generation adenovirus based on the excision of the helper-packaging signal have vectors, while maintaining high transduction efficiency and been generated. Among them, Cre-loxP system is mostly tropism. Nowadays, gutless adenovirus is administered in used, although contamination levels still are 0.1–1% too high different organs, such as the liver, muscle or the central to be used in clinical trials. Recently developed strategies to nervous system achieving high-level and long-term transgene avoid/reduce helper contamination were reviewed. expression in rodents and primates. However, as devoid of all Gene Therapy (2005) 12, S18–S27. doi:10.1038/sj.gt.3302612 Keywords: adenovirus; gutless; helper-dependent vectors; in vivo gene therapy Introduction clinical for more information). Nowadays, adenovirus vectors are applied to treat cancer, monogenic disorders, Gene therapy for most genetic diseases requires expres- vascular diseases and others complications. -

Viral Vectors and Biological Safety
Viral Vectors and Biological Safety Viral vectors are often designed so that they can enter human cells and deliver genes of interest. Viral vectors are usually replication-deficient – genes necessary for replication of the virus are removed from the vector and supplied separately through plasmids, helper virus, or packaging cell lines. There are several biosafety concerns that arise with the use of viral vectors including: 1) Tropism (host range) – viral vectors that can enter (infect) human cells are often used. 2) Replication-deficient viral vectors can gain back the deleted genes required for replication (become replication-competent) through recombination – referred to as replication-competent virus (RCV) breakthroughs. 3) Genes may be expressed in tissues and/or organisms where they are normally not expressed. In the case of some genes such as oncogenes, this could have far-reaching negative consequences. When evaluating safety for use of viral vectors, a number of factors need to be considered including: Risk Group (RG) of the organism; tropism (organism and tissue); route of transmission; whether the virus integrates into the host genome; and the specific gene(s) being introduced. Please contact the Office of Biological Safety (OBS) for more information on physical barriers and safety practices to use with specific viral vectors. This article concentrates on biological barriers that can be employed to improve safety when using viral vectors. Viral vectors frequently used are: • Retrovirus/lentivirus • Adenovirus • Adeno-associated virus (AAV) • Poxvirus • Herpes virus • Alphavirus • Baculovirus Amphotropic murine leukemia virus (MLV) – also called Moloney murine leukemia virus (MMLV) – and adenovirus are common viral vectors used to introduce genes into human cells. -
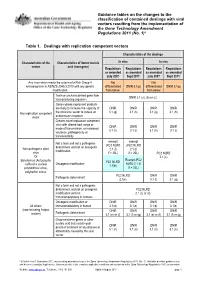
Guidance Tables on the Changes To
Guidance tables on the changes to the classification of contained dealings with viral vectors resulting from the implementation of the Gene Technology Amendment Regulations 2011 (No. 1)* Table 1. Dealings with replication competent vectors Characteristics of the dealings Characteristics of the Characteristics of donor nucleic In vitro In vivo vector acid (transgene) Regulations Regulations Regulations Regulations as amended as amended as amended as amended July 2007 Sept 2011* July 2007 Sept 2011* Any virus which meets the criteria of a Risk Group 4 Not Not microorganism in AS/NZS 2243.3:2010 with any genetic differentiated DNIR 3.1(p) differentiated DNIR 3.1(p) modification from below from below Toxin or uncharacterised gene from DNIR 3.1 (a), (b) or (c) toxin producing organism Genes whose expressed products are likely to increase the capacity of DNIR DNIR DNIR DNIR Any replication competent the virus/viral vector to induce an 3.1 (g) 3.1 (h) 3.1 (g) 3.1 (h) vector autoimmune response Creates novel replication competent virus with altered host range or DNIR DNIR DNIR DNIR mode of transmission, or increased 3.1 (h) 3.1 (i) 3.1 (h) 3.1 (i) virulence, pathogenicity or transmissibility exempt exempt Not a toxin and not a pathogenic (PC2 NLRD (PC2 NLRD determinant and not an oncogenic Non-pathogenic plant 2.1 (f) 2.1 (f) modification virus if > 25L) if > 25L) PC2 NLRD Or 2.1 (c) Exempt (PC2 Baculovirus (Autographa PC1 NLRD Oncogenic modification NLRD 2.1 (f) californica nuclear 1.1(b) polyhedrosis virus), if > 25L) polyhedrin minus PC2 NLRD -

Genome of Phaeocystis Globosa Virus Pgv-16T Highlights the Common Ancestry of the Largest Known DNA Viruses Infecting Eukaryotes
Genome of Phaeocystis globosa virus PgV-16T highlights the common ancestry of the largest known DNA viruses infecting eukaryotes Sebastien Santinia, Sandra Jeudya, Julia Bartolia, Olivier Poirota, Magali Lescota, Chantal Abergela, Valérie Barbeb, K. Eric Wommackc, Anna A. M. Noordeloosd, Corina P. D. Brussaardd,e,1, and Jean-Michel Claveriea,f,1 aStructural and Genomic Information Laboratory, Unité Mixte de Recherche 7256, Centre National de la Recherche Scientifique, Aix-Marseille Université, 13288 Marseille Cedex 9, France; bCommissariat à l’Energie Atomique–Institut de Génomique, 91057 Evry Cedex, France; cDepartment of Plant and Soil Sciences, University of Delaware, Newark, DE 19711; dDepartment of Biological Oceanography, Royal Netherlands Institute for Sea Research, NL-1790 AB Den Burg (Texel), The Netherlands; eAquatic Microbiology, Institute for Biodiversity and Ecosystem Dynamics, University of Amsterdam, Amsterdam, The Netherlands; and fService de Santé Publique et d’Information Médicale, Hôpital de la Timone, Assistance Publique–Hôpitaux de Marseille, FR-13385 Marseille, France Edited by James L. Van Etten, University of Nebraska, Lincoln, NE, and approved May 1, 2013 (received for review February 22, 2013) Large dsDNA viruses are involved in the population control of many viruses: 730 kb and 1.28 Mb for CroV and Megavirus chilensis, globally distributed species of eukaryotic phytoplankton and have respectively. Other studies, targeting virus-specific genes [e.g., a prominent role in bloom termination. The genus Phaeocystis (Hap- DNA polymerase B (8) or capsid proteins (9)] have suggested tophyta, Prymnesiophyceae) includes several high-biomass-forming a close phylogenetic relationship between Mimivirus and several phytoplankton species, such as Phaeocystis globosa, the blooms of giant dsDNA viruses infecting various unicellular algae such as which occur mostly in the coastal zone of the North Atlantic and the Pyramimonas orientalis (Chlorophyta, Prasinophyceae), Phaeocys- North Sea. -

Bacterial Species Library with Validly Published Names (Green Curve); and Sequenced Viral Genomes (Blue)
17 March 2014 ESCMID-ESCAR PGEC MARSEILLE «Ignorance and blindness for a post-modern microbiology ! » © by author ESCMID Online Lecture Library Didier Raoult Marseille - France [email protected] www.mediterranee-infection.com © by author « ESCMIDPostmodern scienceOnline makes Lecture the theory Library of its own evolution as discontinuous, catastrophic, nonrectifiable, paradoxical.» P.97 2 INFECTIONS IN THE XXIST CENTURY Infections in the world : 17 million deaths - 30% - The big 3s : HIV - TB – malaria : no vaccine - Respiratory infections : Etiology? - Digestive infections : Etiology? - Vaccine-prevented infections© by : authorAdhesion - Nosocomial infections : Circuits? - EmergingESCMID infections Online : LectureSource Library - Cancer : Communicable 3 IGNORANCE that decreases but reveals ou ARROGANCE BLINDNES that persists and leads to FALSE DEDUCTIONS © by author and UNVERIFIED PREDICTIONSESCMID Online Lecture Library 4 EMERGING DISEASES IGNORANCE: Major and unique acceleration of knowledge in history Blindness False deductions© by author ESCMID Unverified Online predictions Lecture Library So what ? 5 Viruses: Essential Agents of Life by Günther Witzany © by author ESCMID Online Lecture Library p.64 By F.Rohwer 6 Viruses: Essential Agents of Life by Günther Witzany © by author ESCMID Online Lecture Library p.64 By F.Rohwer 7 PROGRESSES MADE IN MICROBIOLOGY FROM 1979 TO 2012 THANKS TO THE DEVELOPMENT OF NEW TECHNOLOGIES Cultured Sequenced © by author a) the ESCMIDleft ordinate axis refers toOnline the cumulative numbers -

Discovery and Further Studies on Giant Viruses at the IHU Mediterranee Infection That Modified the Perception of the Virosphere
viruses Review Discovery and Further Studies on Giant Viruses at the IHU Mediterranee Infection That Modified the Perception of the Virosphere Clara Rolland 1, Julien Andreani 1, Amina Cherif Louazani 1, Sarah Aherfi 1,3, Rania Francis 1 , 1,2 1, 1 1 Rodrigo Rodrigues , Ludmila Santos Silva y, Dehia Sahmi , Said Mougari , 1 1, 1,2 1,§ 1, Nisrine Chelkha , Meriem Bekliz z, Lorena Silva , Felipe Assis ,Fábio Dornas k, Jacques Yaacoub Bou Khalil 3, Isabelle Pagnier 1,3, Christelle Desnues 1,¶, Anthony Levasseur 1,3, Philippe Colson 1,3,Jônatas Abrahão 1,2 and Bernard La Scola 1,3,* 1 MEPHI, APHM, IRD 198, Aix Marseille Univ, Department of Medicine, IHU-Méditerranée Infection, 13005 Marseille, France; [email protected] (C.R.); [email protected] (J.A.); cherifl[email protected] (A.C.L.); aherfi[email protected] (S.A.); [email protected] (R.F.); [email protected] (R.R.); [email protected] (L.S.S.); [email protected] (D.S.); [email protected] (S.M.); [email protected] (N.C.); [email protected] (M.B.); [email protected] (L.S.); [email protected] (F.A.); [email protected] (F.D.); [email protected] (I.P.); [email protected] (C.D.); [email protected] (A.L.); [email protected] (P.C.); [email protected] (J.A.) 2 Laboratório de Vírus, Instituto de Ciêncas Biológicas, Departamento de Microbiologia, Universidade Federal de Minas Gerais, 31270-901 Belo Horizonte, Brazil 3 IHU IHU-Méditerranée Infection, 13005 Marseille, France; [email protected] * Correspondence: [email protected] Present address: Institut de Biologie Moléculaire et Cellulaire, CNRS UPR9022, Université de Strasbourg, y 67000 Strasbourg, France. -

Virus World As an Evolutionary Network of Viruses and Capsidless Selfish Elements
Virus World as an Evolutionary Network of Viruses and Capsidless Selfish Elements Koonin, E. V., & Dolja, V. V. (2014). Virus World as an Evolutionary Network of Viruses and Capsidless Selfish Elements. Microbiology and Molecular Biology Reviews, 78(2), 278-303. doi:10.1128/MMBR.00049-13 10.1128/MMBR.00049-13 American Society for Microbiology Version of Record http://cdss.library.oregonstate.edu/sa-termsofuse Virus World as an Evolutionary Network of Viruses and Capsidless Selfish Elements Eugene V. Koonin,a Valerian V. Doljab National Center for Biotechnology Information, National Library of Medicine, Bethesda, Maryland, USAa; Department of Botany and Plant Pathology and Center for Genome Research and Biocomputing, Oregon State University, Corvallis, Oregon, USAb Downloaded from SUMMARY ..................................................................................................................................................278 INTRODUCTION ............................................................................................................................................278 PREVALENCE OF REPLICATION SYSTEM COMPONENTS COMPARED TO CAPSID PROTEINS AMONG VIRUS HALLMARK GENES.......................279 CLASSIFICATION OF VIRUSES BY REPLICATION-EXPRESSION STRATEGY: TYPICAL VIRUSES AND CAPSIDLESS FORMS ................................279 EVOLUTIONARY RELATIONSHIPS BETWEEN VIRUSES AND CAPSIDLESS VIRUS-LIKE GENETIC ELEMENTS ..............................................280 Capsidless Derivatives of Positive-Strand RNA Viruses....................................................................................................280 -

Viral Vectors
OCCUPATIONAL HEALTH CONSIDERATIONS FOR WORK WITH LENTIVIRAL VECTORS GARY R. FUJIMOTO, M.D. OCCUPATIONAL MEDICINE CONSULTANT OCTOBER 6, 2014 Disclosure: Lecture includes off-label use of antiretroviral medications VIRAL VECTORS Definition: Viruses engineered to deliver foreign genetic material (transgene) to cells Many viral vectors deliver the genetic material into the host cells but not into the host genome where the virus replicates (unless replication incompetent) Retroviral and lentiviral vectors deliver genetic transgenes into the host chromosomes LENTIVIRAL VECTORS Human immunodeficiency virus (HIV) is a lentivirus that infects both dividing and non-dividing cells Use of the HIV virus as a viral vector has required the reengineering of the virus to achieve safe gene transfer Since HIV normally targets CD4 cells, replacing the HIV envelope gene with vesicular stomatitis virus glycoprotein (VSV-G) expands the infectious range of the vector and modes of transmission LENTIVIRAL VECTORS Remember: replication deficient lentiviral vectors integrate the vector into the host chromosomes 3rd and 4th generation constructs unlikely to become replication competent with enhanced safety due to self- inactivating vectors (however, consider present or future HIV infection) Replication deficient lentiviral vectors should be regarded as single-event infectious agents Many researchers regard these agents as relatively benign although transgene integration does occur with generally unknown effects LENTIVIRAL OCCUPATIONAL EXPOSURES Lentiviral -

Viral Vectors
505 Oldham Court Lexington, KY 40502 Phone: (859) 257-1049 Fax: (859) 323-3838 E-Mail: [email protected] http://ehs.uky.edu/biosafety VIRAL VECTORS WHAT IS A VIRAL VECTOR? Viral vectors work like a “nanosyringe” to deliver nucleic acid to a target. They are often more efficient than other transfection methods, are useful for whole organism studies, have a relatively low toxicity, and are a likely route for human gene transfer. All viral vectors require a host for replication. The production of a viral vector is typically separated from the ability of the viral vector to infect cells. While viral vectors are not typically considered infectious agents, they do maintain their ability to “infect” cells. Viral vectors just don’t replicate (although there are some replicating viral vectors in use) under experimental conditions. An HIV- based lentiviral vector no longer possesses the ability to infect an individual with HIV, but it does maintain the ability to enter a cell and express genetic information. This is why viral vectors are useful, but also require caution. If a viral vector can transfect a human cell line on a plate, it can also transfect YOUR cells if accidentally exposed. SAFETY CONSIDERATIONS FOR ALL VIRAL VECTORS When utilizing ANY viral vector, the following questions must be addressed… 1. What potential does your method of viral vector production have to generate a replication competent virus? a. Generation of viral vector refers to the number of recombination events required to form a replication competent virus. For example, if you’re using a lentivirus that is split up between 4 plasmids (gag/pol, VSV-g, rev, transgene), 3 recombination events must take place to create a replication competent virus, therefore you are using a 3rd generation lentiviral vector. -

RNA Viruses As Tools in Gene Therapy and Vaccine Development
G C A T T A C G G C A T genes Review RNA Viruses as Tools in Gene Therapy and Vaccine Development Kenneth Lundstrom PanTherapeutics, Rte de Lavaux 49, CH1095 Lutry, Switzerland; [email protected]; Tel.: +41-79-776-6351 Received: 31 January 2019; Accepted: 21 February 2019; Published: 1 March 2019 Abstract: RNA viruses have been subjected to substantial engineering efforts to support gene therapy applications and vaccine development. Typically, retroviruses, lentiviruses, alphaviruses, flaviviruses rhabdoviruses, measles viruses, Newcastle disease viruses, and picornaviruses have been employed as expression vectors for treatment of various diseases including different types of cancers, hemophilia, and infectious diseases. Moreover, vaccination with viral vectors has evaluated immunogenicity against infectious agents and protection against challenges with pathogenic organisms. Several preclinical studies in animal models have confirmed both immune responses and protection against lethal challenges. Similarly, administration of RNA viral vectors in animals implanted with tumor xenografts resulted in tumor regression and prolonged survival, and in some cases complete tumor clearance. Based on preclinical results, clinical trials have been conducted to establish the safety of RNA virus delivery. Moreover, stem cell-based lentiviral therapy provided life-long production of factor VIII potentially generating a cure for hemophilia A. Several clinical trials on cancer patients have generated anti-tumor activity, prolonged survival, and