Lentiviral Vectors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Non-Primate Lentiviral Vectors and Their Applications in Gene Therapy for Ocular Disorders
viruses Review Non-Primate Lentiviral Vectors and Their Applications in Gene Therapy for Ocular Disorders Vincenzo Cavalieri 1,2,* ID , Elena Baiamonte 3 and Melania Lo Iacono 3 1 Department of Biological, Chemical and Pharmaceutical Sciences and Technologies (STEBICEF), University of Palermo, Viale delle Scienze Edificio 16, 90128 Palermo, Italy 2 Advanced Technologies Network (ATeN) Center, University of Palermo, Viale delle Scienze Edificio 18, 90128 Palermo, Italy 3 Campus of Haematology Franco e Piera Cutino, Villa Sofia-Cervello Hospital, 90146 Palermo, Italy; [email protected] (E.B.); [email protected] (M.L.I.) * Correspondence: [email protected] Received: 30 April 2018; Accepted: 7 June 2018; Published: 9 June 2018 Abstract: Lentiviruses have a number of molecular features in common, starting with the ability to integrate their genetic material into the genome of non-dividing infected cells. A peculiar property of non-primate lentiviruses consists in their incapability to infect and induce diseases in humans, thus providing the main rationale for deriving biologically safe lentiviral vectors for gene therapy applications. In this review, we first give an overview of non-primate lentiviruses, highlighting their common and distinctive molecular characteristics together with key concepts in the molecular biology of lentiviruses. We next examine the bioengineering strategies leading to the conversion of lentiviruses into recombinant lentiviral vectors, discussing their potential clinical applications in ophthalmological research. Finally, we highlight the invaluable role of animal organisms, including the emerging zebrafish model, in ocular gene therapy based on non-primate lentiviral vectors and in ophthalmology research and vision science in general. Keywords: FIV; EIAV; BIV; JDV; VMV; CAEV; lentiviral vector; gene therapy; ophthalmology; zebrafish 1. -

Mobile Genetic Elements in Streptococci
Curr. Issues Mol. Biol. (2019) 32: 123-166. DOI: https://dx.doi.org/10.21775/cimb.032.123 Mobile Genetic Elements in Streptococci Miao Lu#, Tao Gong#, Anqi Zhang, Boyu Tang, Jiamin Chen, Zhong Zhang, Yuqing Li*, Xuedong Zhou* State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu, PR China. #Miao Lu and Tao Gong contributed equally to this work. *Address correspondence to: [email protected], [email protected] Abstract Streptococci are a group of Gram-positive bacteria belonging to the family Streptococcaceae, which are responsible of multiple diseases. Some of these species can cause invasive infection that may result in life-threatening illness. Moreover, antibiotic-resistant bacteria are considerably increasing, thus imposing a global consideration. One of the main causes of this resistance is the horizontal gene transfer (HGT), associated to gene transfer agents including transposons, integrons, plasmids and bacteriophages. These agents, which are called mobile genetic elements (MGEs), encode proteins able to mediate DNA movements. This review briefly describes MGEs in streptococci, focusing on their structure and properties related to HGT and antibiotic resistance. caister.com/cimb 123 Curr. Issues Mol. Biol. (2019) Vol. 32 Mobile Genetic Elements Lu et al Introduction Streptococci are a group of Gram-positive bacteria widely distributed across human and animals. Unlike the Staphylococcus species, streptococci are catalase negative and are subclassified into the three subspecies alpha, beta and gamma according to the partial, complete or absent hemolysis induced, respectively. The beta hemolytic streptococci species are further classified by the cell wall carbohydrate composition (Lancefield, 1933) and according to human diseases in Lancefield groups A, B, C and G. -

Gutless Adenovirus: Last-Generation Adenovirus for Gene Therapy
Gene Therapy (2005) 12, S18–S27 & 2005 Nature Publishing Group All rights reserved 0969-7128/05 $30.00 www.nature.com/gt CONFERENCE PAPER Gutless adenovirus: last-generation adenovirus for gene therapy R Alba1, A Bosch1 and M Chillon1,2 1Gene Therapy Laboratory, Department of Biochemistry and Molecular Biology, Center of Animal Biotechnology and Gene Therapy (CBATEG), Universitat Auto`noma de Barcelona, Bellaterra, Spain; and 2Institut Catala` de Recerca i Estudis Avanc¸ats (ICREA), Barcelona, Spain Last-generation adenovirus vectors, also called helper-depen- viral coding regions, gutless vectors require viral proteins dent or gutless adenovirus, are very attractive for gene therapy supplied in trans by a helper virus. To remove contamination because the associated in vivo immune response is highly by a helper virus from the final preparation, different systems reduced compared to first- and second-generation adenovirus based on the excision of the helper-packaging signal have vectors, while maintaining high transduction efficiency and been generated. Among them, Cre-loxP system is mostly tropism. Nowadays, gutless adenovirus is administered in used, although contamination levels still are 0.1–1% too high different organs, such as the liver, muscle or the central to be used in clinical trials. Recently developed strategies to nervous system achieving high-level and long-term transgene avoid/reduce helper contamination were reviewed. expression in rodents and primates. However, as devoid of all Gene Therapy (2005) 12, S18–S27. doi:10.1038/sj.gt.3302612 Keywords: adenovirus; gutless; helper-dependent vectors; in vivo gene therapy Introduction clinical for more information). Nowadays, adenovirus vectors are applied to treat cancer, monogenic disorders, Gene therapy for most genetic diseases requires expres- vascular diseases and others complications. -

LENTIVIRUS and LENTIVIRAL VECTORS
LENTIVIRUS and LENTIVIRAL VECTORS Risk Group: 3 I. Background and Health Hazards Lentiviruses are a subset of retroviruses that have the ability to integrate into host chromosomes and to infect non-dividing cells, and include human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) which can infect humans. Another commonly used lentivirus that is infectious to animals, but not humans, is feline immunodeficiency virus (FIV). Lentiviral vectors consist of recombinant or synthetic nucleic acid sequences and HIV or other lentivirus- based viral packaging and regulatory sequences flanked by either wild-type or chimeric long terminal repeat (LTR) regions. Use of these vector systems is particularly desirable because of their ability to integrate transgenes into dividing, as well as, non-dividing cells. However, there are risks associated with working with lentiviral vectors and these must be carefully evaluated. The two major risks are: 1) the potential generation of replication competent lentivirus (RCL); and 2) the potential for oncogenesis. These risks are largely based upon the vector system used and the transgene insert encoded by the vector. Therefore, it is imperative that prior to utilizing a lentiviral vector system, a risk assessment must be reviewed and documented. Also, because construction and/or use of lentiviral vectors falls under the definition of r/sNA research as outlined in the NIH Guidelines, possession must be reported in the workplace’s biological agent inventory and experiments must be submitted for review and approval by the site IBC prior to initiation. II. MODES OF TRANSMISSION Lentivirus is transmissible through injection, ingestion, exposure to broken skin or contact with mucous membranes of the eyes, nose and mouth. -

Viral Vectors and Biological Safety
Viral Vectors and Biological Safety Viral vectors are often designed so that they can enter human cells and deliver genes of interest. Viral vectors are usually replication-deficient – genes necessary for replication of the virus are removed from the vector and supplied separately through plasmids, helper virus, or packaging cell lines. There are several biosafety concerns that arise with the use of viral vectors including: 1) Tropism (host range) – viral vectors that can enter (infect) human cells are often used. 2) Replication-deficient viral vectors can gain back the deleted genes required for replication (become replication-competent) through recombination – referred to as replication-competent virus (RCV) breakthroughs. 3) Genes may be expressed in tissues and/or organisms where they are normally not expressed. In the case of some genes such as oncogenes, this could have far-reaching negative consequences. When evaluating safety for use of viral vectors, a number of factors need to be considered including: Risk Group (RG) of the organism; tropism (organism and tissue); route of transmission; whether the virus integrates into the host genome; and the specific gene(s) being introduced. Please contact the Office of Biological Safety (OBS) for more information on physical barriers and safety practices to use with specific viral vectors. This article concentrates on biological barriers that can be employed to improve safety when using viral vectors. Viral vectors frequently used are: • Retrovirus/lentivirus • Adenovirus • Adeno-associated virus (AAV) • Poxvirus • Herpes virus • Alphavirus • Baculovirus Amphotropic murine leukemia virus (MLV) – also called Moloney murine leukemia virus (MMLV) – and adenovirus are common viral vectors used to introduce genes into human cells. -
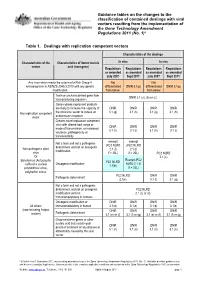
Guidance Tables on the Changes To
Guidance tables on the changes to the classification of contained dealings with viral vectors resulting from the implementation of the Gene Technology Amendment Regulations 2011 (No. 1)* Table 1. Dealings with replication competent vectors Characteristics of the dealings Characteristics of the Characteristics of donor nucleic In vitro In vivo vector acid (transgene) Regulations Regulations Regulations Regulations as amended as amended as amended as amended July 2007 Sept 2011* July 2007 Sept 2011* Any virus which meets the criteria of a Risk Group 4 Not Not microorganism in AS/NZS 2243.3:2010 with any genetic differentiated DNIR 3.1(p) differentiated DNIR 3.1(p) modification from below from below Toxin or uncharacterised gene from DNIR 3.1 (a), (b) or (c) toxin producing organism Genes whose expressed products are likely to increase the capacity of DNIR DNIR DNIR DNIR Any replication competent the virus/viral vector to induce an 3.1 (g) 3.1 (h) 3.1 (g) 3.1 (h) vector autoimmune response Creates novel replication competent virus with altered host range or DNIR DNIR DNIR DNIR mode of transmission, or increased 3.1 (h) 3.1 (i) 3.1 (h) 3.1 (i) virulence, pathogenicity or transmissibility exempt exempt Not a toxin and not a pathogenic (PC2 NLRD (PC2 NLRD determinant and not an oncogenic Non-pathogenic plant 2.1 (f) 2.1 (f) modification virus if > 25L) if > 25L) PC2 NLRD Or 2.1 (c) Exempt (PC2 Baculovirus (Autographa PC1 NLRD Oncogenic modification NLRD 2.1 (f) californica nuclear 1.1(b) polyhedrosis virus), if > 25L) polyhedrin minus PC2 NLRD -

Lentivirus Vector Fact Sheet
LENTIVIRUS VECTOR FACT SHEET LENTIVIRUSES: Lentiviral vector constructs are derived from HIV and are therefore highly efficient vehicles for in vivo gene delivery. Use of these vector systems is particularly desirable because of their ability to integrate transgenes into dividing, as well as, non-dividing cells. However, there are risks associated with working with lentiviral vectors and these must be carefully evaluated. The two major risks are: 1) the potential generation of replication competent virus; and 2) the potential for oncogenesis through insertional mutagenesis. These risks are largely based upon the vector system used and the transgene insert encoded by the vector. Therefore, it is imperative that prior to utilizing a lentiviral vector system, a risk assessment must be completed and documented. Also, because construction and/or use of lentiviral vectors falls under the definition of rDNA research as outlined in the NIH Recombinant DNA Guidelines, possession must be reported in the workplace’s biological agent inventory and experiments must be submitted for review and approval by the site IBC prior to initiation. Typically, lentiviral vectors may be safely handled using either BSL-2 or BSL-2 enhanced controls depending upon the risk assessment. LENTIVIRAL VECTORS: Lentiviruses can infect not only dividing, but also non-dividing host cells, because their pre-integration complex (virus “shell”) can get through the intact membrane of the nucleus of the target cell. Lentiviral vectors can be used to provide highly effective gene therapy as they can provide long-term expression of the vectored transgene in target cells. First-generation lentiviral vectors were manufactured using a single vector packaging system that contained all HIV genes, except the envelope (env) gene, in one plasmid. -

Feline Foamy Virus-Based Vectors: Advantages of an Authentic Animal Model
Viruses 2013, 5, 1702-1718; doi:10.3390/v5071702 OPEN ACCESS viruses ISSN 1999-4915 www.mdpi.com/journal/viruses Review Feline Foamy Virus-Based Vectors: Advantages of an Authentic Animal Model †,‡ † Weibin Liu , Janet Lei , Yang Liu, Dragana Slavkovic Lukic, Ann-Mareen Räthe, # Qiuying Bao, Timo Kehl, Anne Bleiholder, Torsten Hechler and Martin Löchelt * Department of Genome Modifications, Research Program Infection and Cancer, German Cancer Research Center, Im Neuenheimer Feld 242, 69120 Heidelberg, Germany; E-Mails: [email protected] (W.L.); [email protected] (J.L.); [email protected] (Y.L.); [email protected] (D.S.L.); [email protected] (A.-M.R.); [email protected] (Q.B.); [email protected] (T.K.); [email protected] (T.H.) † These authors contributed equally to this work. ‡ Current address: Department of Biochemistry and Molecular Biology, Mayo Clinic College of Medicine, 200 First Street SW, Rochester, MN 55905, USA. # Current address: Department of Biochemistry, Heidelberg Pharma GmbH, Schriesheimer Str. 101, 68526 Ladenburg, Germany. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +49-6221-424933; Fax: +49-6221-424932. Received: 1 March 2013; in revised form: 13 June 2013 / Accepted: 25 June 2013 / Published: 12 July 2013 Abstract: New-generation retroviral vectors have potential applications in vaccination and gene therapy. Foamy viruses are particularly interesting as vectors, because they are not associated to any disease. Vector research is mainly based on primate foamy viruses (PFV), but cats are an alternative animal model, due to their smaller size and the existence of a cognate feline foamy virus (FFV). -

Protocol and Reagents for Pseudotyping Lentiviral Particles with SARS-Cov-2 Spike Protein for Neutralization Assays
bioRxiv preprint doi: https://doi.org/10.1101/2020.04.20.051219; this version posted April 20, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 of 15 Protocol and reagents for pseudotyping lentiviral particles with SARS-CoV-2 Spike protein for neutralization assays Katharine H.D. Crawford 1,2,3, Rachel Eguia 1, Adam S. Dingens 1, Andrea N. Loes 1, Keara D. Malone 1, Caitlin R. Wolf 4, Helen Y. Chu 4, M. Alejandra Tortorici 5,6, David Veesler 5, Michael Murphy 7, Deleah Pettie 7, Neil P. King 5,7, Alejandro B. Balazs 8, and Jesse D. Bloom 1,2,9,* 1 Division of Basic Sciences and Computational Biology Program, Fred Hutchinson Cancer Research Center, Seattle, WA 98109, USA; [email protected] (K.D.C.), [email protected] (R.E.), [email protected] (A.S.D.), [email protected] (K.M.), [email protected] (A.N.L.) 2 Department of Genome Sciences, University of Washington, Seattle, WA 98195, USA 3 Medical Scientist Training Program, University of Washington, Seattle, WA 98195, USA 4 Division of Allergy and Infectious Diseases, University of Washington, Seattle, WA 98195, USA; [email protected] (C.R.W.), [email protected] (H.Y.C.) 5 Department of Biochemistry, University of Washington, Seattle, WA 98109, USA; [email protected] (M.A.T.), [email protected] (D.V.), [email protected] (M.M.), [email protected] (D.P.), [email protected] (N.P.K.) 6 Institute -

Lentivirus-Mediated Gene Transfer to the Respiratory Epithelium: a Promising Approach to Gene Therapy of Cystic fibrosis
Gene Therapy (2004) 11, S67–S75 & 2004 Nature Publishing Group All rights reserved 0969-7128/04 $30.00 www.nature.com/gt REVIEW Lentivirus-mediated gene transfer to the respiratory epithelium: a promising approach to gene therapy of cystic fibrosis E Copreni, M Penzo, S Carrabino and M Conese Institute for Experimental Treatment of Cystic Fibrosis, HS Raffaele, Milano, Italy Gene therapy of cystic fibrosis (CF) lung disease needs logous envelopes are the strategies currently used to highly efficient delivery and long-lasting complementation overcome the paucity of specific viral receptors on the apical of the CFTR (cystic fibrosis transmembrane conductance surface of airway epithelial cells and to reach the basolateral regulator) gene into the respiratory epithelium. The develop- surface receptors. Preclinical studies on CF mice, demon- ment of lentiviral vectors has been a recent advance in the strating complementation of the CF defect, offer hope that field of gene transfer and therapy. These integrating vectors lentivirus gene therapy can be translated into an effective appear to be promising vehicles for gene delivery into treatment of CF lung disease. Besides a direct targeting of respiratory epithelial cells by virtue of their ability to infect the stem/progenitor niche(s) in the CF airways, an alternative nondividing cells and mediate long-term persistence of approach may envision homing of hematopoietic stem cells transgene expression. Studies in human airway tissues and engineered to express the CFTR gene by lentiviral vectors. animal models have highlighted the possibility of achieving In the context of lentivirus-mediated CFTR gene transfer gene expression by lentiviral vectors, which outlasted the to the CF airways, biosafety aspects should be of primary normal lifespan of the respiratory epithelium, indicating concern. -

Study of Chikungunya Virus Entry and Host Response to Infection Marie Cresson
Study of chikungunya virus entry and host response to infection Marie Cresson To cite this version: Marie Cresson. Study of chikungunya virus entry and host response to infection. Virology. Uni- versité de Lyon; Institut Pasteur of Shanghai. Chinese Academy of Sciences, 2019. English. NNT : 2019LYSE1050. tel-03270900 HAL Id: tel-03270900 https://tel.archives-ouvertes.fr/tel-03270900 Submitted on 25 Jun 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. N°d’ordre NNT : 2019LYSE1050 THESE de DOCTORAT DE L’UNIVERSITE DE LYON opérée au sein de l’Université Claude Bernard Lyon 1 Ecole Doctorale N° 341 – E2M2 Evolution, Ecosystèmes, Microbiologie, Modélisation Spécialité de doctorat : Biologie Discipline : Virologie Soutenue publiquement le 15/04/2019, par : Marie Cresson Study of chikungunya virus entry and host response to infection Devant le jury composé de : Choumet Valérie - Chargée de recherche - Institut Pasteur Paris Rapporteure Meng Guangxun - Professeur - Institut Pasteur Shanghai Rapporteur Lozach Pierre-Yves - Chargé de recherche - CHU d'Heidelberg Rapporteur Kretz Carole - Professeure - Université Claude Bernard Lyon 1 Examinatrice Roques Pierre - Directeur de recherche - CEA Fontenay-aux-Roses Examinateur Maisse-Paradisi Carine - Chargée de recherche - INRA Directrice de thèse Lavillette Dimitri - Professeur - Institut Pasteur Shanghai Co-directeur de thèse 2 UNIVERSITE CLAUDE BERNARD - LYON 1 Président de l’Université M. -

Molecular Analysis of the Complete Genome of a Simian Foamy Virus Infecting Hylobates Pileatus (Pileated Gibbon) Reveals Ancient Co-Evolution with Lesser Apes
viruses Article Molecular Analysis of the Complete Genome of a Simian Foamy Virus Infecting Hylobates pileatus (pileated gibbon) Reveals Ancient Co-Evolution with Lesser Apes Anupama Shankar 1, Samuel D. Sibley 2, Tony L. Goldberg 2 and William M. Switzer 1,* 1 Laboratory Branch, Division of HIV/AIDS Prevention, Center for Disease Control and Prevention, Atlanta, GA 30329, USA 2 Department of Pathobiological Sciences, School of Veterinary Medicine, University of Wisconsin-Madison, Madison, WI 53706, USA * Correspondence: [email protected]; Tel.: +1-404-639-2019 Received: 1 April 2019; Accepted: 30 June 2019; Published: 3 July 2019 Abstract: Foamy viruses (FVs) are complex retroviruses present in many mammals, including nonhuman primates, where they are called simian foamy viruses (SFVs). SFVs can zoonotically infect humans, but very few complete SFV genomes are available, hampering the design of diagnostic assays. Gibbons are lesser apes widespread across Southeast Asia that can be infected with SFV, but only two partial SFV sequences are currently available. We used a metagenomics approach with next-generation sequencing of nucleic acid extracted from the cell culture of a blood specimen from a lesser ape, the pileated gibbon (Hylobates pileatus), to obtain the complete SFVhpi_SAM106 genome. We used Bayesian analysis to co-infer phylogenetic relationships and divergence dates. SFVhpi_SAM106 is ancestral to other ape SFVs with a divergence date of ~20.6 million years ago, reflecting ancient co-evolution of the host and SFVhpi_SAM106. Analysis of the complete SFVhpi_SAM106 genome shows that it has the same genetic architecture as other SFVs but has the longest recorded genome (13,885-nt) due to a longer long terminal repeat region (2,071 bp).