Retro-Viral Vectors
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Mobile Genetic Elements in Streptococci
Curr. Issues Mol. Biol. (2019) 32: 123-166. DOI: https://dx.doi.org/10.21775/cimb.032.123 Mobile Genetic Elements in Streptococci Miao Lu#, Tao Gong#, Anqi Zhang, Boyu Tang, Jiamin Chen, Zhong Zhang, Yuqing Li*, Xuedong Zhou* State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu, PR China. #Miao Lu and Tao Gong contributed equally to this work. *Address correspondence to: [email protected], [email protected] Abstract Streptococci are a group of Gram-positive bacteria belonging to the family Streptococcaceae, which are responsible of multiple diseases. Some of these species can cause invasive infection that may result in life-threatening illness. Moreover, antibiotic-resistant bacteria are considerably increasing, thus imposing a global consideration. One of the main causes of this resistance is the horizontal gene transfer (HGT), associated to gene transfer agents including transposons, integrons, plasmids and bacteriophages. These agents, which are called mobile genetic elements (MGEs), encode proteins able to mediate DNA movements. This review briefly describes MGEs in streptococci, focusing on their structure and properties related to HGT and antibiotic resistance. caister.com/cimb 123 Curr. Issues Mol. Biol. (2019) Vol. 32 Mobile Genetic Elements Lu et al Introduction Streptococci are a group of Gram-positive bacteria widely distributed across human and animals. Unlike the Staphylococcus species, streptococci are catalase negative and are subclassified into the three subspecies alpha, beta and gamma according to the partial, complete or absent hemolysis induced, respectively. The beta hemolytic streptococci species are further classified by the cell wall carbohydrate composition (Lancefield, 1933) and according to human diseases in Lancefield groups A, B, C and G. -

Characterization of the Matrix Proteins of the Fish Rhabdovirus, Infectious Hematopoietic Necrosis Virus
AN ABSTRACT OF THE THESIS OF Patricia A. Ormonde for the degree of Master of Science presented on April 14. 1995. Title: Characterization of the Matrix Proteins of the Fish Rhabdovinis, Infectious Hematopoietic Necrosis Virus. Redacted for Privacy Abstract approved: Jo-Ann C. ong Infectious hematopoietic necrosis virus (1HNV) is an important fish pathogen enzootic in salmon and trout populations of the Pacific Northwestern United States. Occasional epizootics in fish hatcheries can result in devastating losses of fish stocks. The complete nucleotide sequence of IHNV has not yet been determined. This knowledge is the first step towards understanding the roles viral proteins play in IHNV infection, and is necessary for determining the relatedness of IHNV to other rhabdoviruses. The glycoprotein, nucleocapsid and non-virion genes of IHNV have been described previously; however, at the initiation of this study, very little was known about the matrix protein genes. Rhabdoviral matrix proteins have been found to be important in viral transcription and virion assembly. This thesis describes the preliminary characterization of the M1 and M2 matrix proteins of IHNV. In addition, the trout humoral immune response to M1 and M2 proteins expressed from plasmid DNA injected into the fish was investigated. This work may prove useful in designing future vaccines against IHN. The sequences of M1 phosphoprotein and M2 matrix protein genes of IHNV were determined from both genomic and mRNA clones. Analysis of the sequences indicated that the predicted open reading frame of M1 gene encoded a 230 amino acid protein with a estimated molecular weight of 25.6 kDa. Further analysis revealed a second open reading frame encoding a 42 amino acid protein with a calculated molecular weight of 4.8 kDa. -

Gutless Adenovirus: Last-Generation Adenovirus for Gene Therapy
Gene Therapy (2005) 12, S18–S27 & 2005 Nature Publishing Group All rights reserved 0969-7128/05 $30.00 www.nature.com/gt CONFERENCE PAPER Gutless adenovirus: last-generation adenovirus for gene therapy R Alba1, A Bosch1 and M Chillon1,2 1Gene Therapy Laboratory, Department of Biochemistry and Molecular Biology, Center of Animal Biotechnology and Gene Therapy (CBATEG), Universitat Auto`noma de Barcelona, Bellaterra, Spain; and 2Institut Catala` de Recerca i Estudis Avanc¸ats (ICREA), Barcelona, Spain Last-generation adenovirus vectors, also called helper-depen- viral coding regions, gutless vectors require viral proteins dent or gutless adenovirus, are very attractive for gene therapy supplied in trans by a helper virus. To remove contamination because the associated in vivo immune response is highly by a helper virus from the final preparation, different systems reduced compared to first- and second-generation adenovirus based on the excision of the helper-packaging signal have vectors, while maintaining high transduction efficiency and been generated. Among them, Cre-loxP system is mostly tropism. Nowadays, gutless adenovirus is administered in used, although contamination levels still are 0.1–1% too high different organs, such as the liver, muscle or the central to be used in clinical trials. Recently developed strategies to nervous system achieving high-level and long-term transgene avoid/reduce helper contamination were reviewed. expression in rodents and primates. However, as devoid of all Gene Therapy (2005) 12, S18–S27. doi:10.1038/sj.gt.3302612 Keywords: adenovirus; gutless; helper-dependent vectors; in vivo gene therapy Introduction clinical for more information). Nowadays, adenovirus vectors are applied to treat cancer, monogenic disorders, Gene therapy for most genetic diseases requires expres- vascular diseases and others complications. -

Parallels Among Positive-Strand RNA Viruses, Reverse-Transcribing Viruses and Double-Stranded RNA Viruses
REVIEWS Parallels among positive-strand RNA viruses, reverse-transcribing viruses and double-stranded RNA viruses Paul Ahlquist Abstract | Viruses are divided into seven classes on the basis of differing strategies for storing and replicating their genomes through RNA and/or DNA intermediates. Despite major differences among these classes, recent results reveal that the non-virion, intracellular RNA- replication complexes of some positive-strand RNA viruses share parallels with the structure, assembly and function of the replicative cores of extracellular virions of reverse-transcribing viruses and double-stranded RNA viruses. Therefore, at least four of seven principal virus classes share several underlying features in genome replication and might have emerged from common ancestors. This has implications for virus function, evolution and control. Positive-strand RNA virus Despite continuing advances, established and emerging ssRNA or dsRNA. Other viruses replicate by intercon- A virus, the infectious virions of viruses remain major causes of human disease, with verting their genomes between RNA and DNA. The viri- which contain the genome in a dramatic costs in mortality, morbidity and economic ons of such reverse-transcribing viruses always initially single-stranded, messenger- terms. In addition to acute diseases, viruses cause at package the RNA forms of their genomes, and either sense RNA form. least 15–20% of human cancers1,2 and are implicated in might (for example, hepadnaviruses and foamy retro- neurological and other chronic disorders. One of many viruses) or might not (for example, orthoretroviruses) challenges in controlling viruses and virus-mediated dis- reverse-transcribe the RNA into DNA before the virion eases is that viruses show an amazing diversity in basic exits the initially infected producer cell. -

Viral Vectors and Biological Safety
Viral Vectors and Biological Safety Viral vectors are often designed so that they can enter human cells and deliver genes of interest. Viral vectors are usually replication-deficient – genes necessary for replication of the virus are removed from the vector and supplied separately through plasmids, helper virus, or packaging cell lines. There are several biosafety concerns that arise with the use of viral vectors including: 1) Tropism (host range) – viral vectors that can enter (infect) human cells are often used. 2) Replication-deficient viral vectors can gain back the deleted genes required for replication (become replication-competent) through recombination – referred to as replication-competent virus (RCV) breakthroughs. 3) Genes may be expressed in tissues and/or organisms where they are normally not expressed. In the case of some genes such as oncogenes, this could have far-reaching negative consequences. When evaluating safety for use of viral vectors, a number of factors need to be considered including: Risk Group (RG) of the organism; tropism (organism and tissue); route of transmission; whether the virus integrates into the host genome; and the specific gene(s) being introduced. Please contact the Office of Biological Safety (OBS) for more information on physical barriers and safety practices to use with specific viral vectors. This article concentrates on biological barriers that can be employed to improve safety when using viral vectors. Viral vectors frequently used are: • Retrovirus/lentivirus • Adenovirus • Adeno-associated virus (AAV) • Poxvirus • Herpes virus • Alphavirus • Baculovirus Amphotropic murine leukemia virus (MLV) – also called Moloney murine leukemia virus (MMLV) – and adenovirus are common viral vectors used to introduce genes into human cells. -
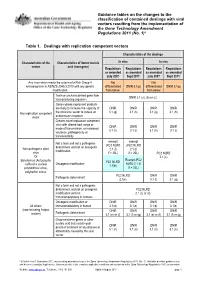
Guidance Tables on the Changes To
Guidance tables on the changes to the classification of contained dealings with viral vectors resulting from the implementation of the Gene Technology Amendment Regulations 2011 (No. 1)* Table 1. Dealings with replication competent vectors Characteristics of the dealings Characteristics of the Characteristics of donor nucleic In vitro In vivo vector acid (transgene) Regulations Regulations Regulations Regulations as amended as amended as amended as amended July 2007 Sept 2011* July 2007 Sept 2011* Any virus which meets the criteria of a Risk Group 4 Not Not microorganism in AS/NZS 2243.3:2010 with any genetic differentiated DNIR 3.1(p) differentiated DNIR 3.1(p) modification from below from below Toxin or uncharacterised gene from DNIR 3.1 (a), (b) or (c) toxin producing organism Genes whose expressed products are likely to increase the capacity of DNIR DNIR DNIR DNIR Any replication competent the virus/viral vector to induce an 3.1 (g) 3.1 (h) 3.1 (g) 3.1 (h) vector autoimmune response Creates novel replication competent virus with altered host range or DNIR DNIR DNIR DNIR mode of transmission, or increased 3.1 (h) 3.1 (i) 3.1 (h) 3.1 (i) virulence, pathogenicity or transmissibility exempt exempt Not a toxin and not a pathogenic (PC2 NLRD (PC2 NLRD determinant and not an oncogenic Non-pathogenic plant 2.1 (f) 2.1 (f) modification virus if > 25L) if > 25L) PC2 NLRD Or 2.1 (c) Exempt (PC2 Baculovirus (Autographa PC1 NLRD Oncogenic modification NLRD 2.1 (f) californica nuclear 1.1(b) polyhedrosis virus), if > 25L) polyhedrin minus PC2 NLRD -

Theory of an Immune System Retrovirus
Proc. Nati. Acad. Sci. USA Vol. 83, pp. 9159-9163, December 1986 Medical Sciences Theory of an immune system retrovirus (human immunodeficiency virus/acquired immune deficiency syndrome) LEON N COOPER Physics Department and Center for Neural Science, Brown University, Providence, RI 02912 Contributed by Leon N Cooper, July 23, 1986 ABSTRACT Human immunodeficiency virus (HIV; for- initiates clonal expansion, sustained by interleukin 2 and y merly known as human T-cell lymphotropic virus type interferon. Ill/lymphadenopathy-associated virus, HTLV-Ill/LAV), the I first give a brief sketch of these events in a linked- retrovirus that infects T4-positive (helper) T cells of the interaction model in which it is assumed that antigen-specific immune system, has been implicated as the agent responsible T cells must interact with the B-cell-processed virus to for the acquired immune deficiency syndrome. In this paper, initiate clonal expansion (2). I then assume that virus-specific I contrast the growth of a "normal" virus with what I call an antibody is the major component ofimmune system response immune system retrovirus: a retrovirus that attacks the T4- that limits virus spread. As will be seen, the details of these positive T cells of the immune system. I show that remarkable assumptions do not affect the qualitative features of my interactions with other infections as well as strong virus conclusions. concentration dependence are general properties of immune Linked-Interaction Model for Clonal Expansion of Lympho- system retroviruses. Some of the consequences of these ideas cytes. Let X be the concentration of normal infecting virus are compared with observations. -

Discovery of HTLV-1, the First Human Retrovirus, Its Unique Regulatory Mechanisms, and Insights Into Pathogenesis
Oncogene (2005) 24, 5931–5937 & 2005 Nature Publishing Group All rights reserved 0950-9232/05 $30.00 www.nature.com/onc Discovery of HTLV-1, the first human retrovirus, its unique regulatory mechanisms, and insights into pathogenesis Mitsuaki Yoshida*,1 1Banyu Tsukuba Research Institute, Banyu Pharmaceutical Co., Ltd., 3 Ohkubo, Tsukuba, Ibaraki 300-2611, Japan I briefly review the discovery and characterization of the In Japan something was there, but it was not clear first human retrovirus, human T-cell leukemia virus type 1, focusing on contributions from Japanese researchers. Discovery of ATL The unique regulatory mechanisms for the viral regulation The first key event for human retrovirus was the with Tax and Rex, etiology of ATL and possible discovery of adult T-cell leukemia (ATL) by Kiyoshi leukemogenic mechanism with Tax are also discussed Takatsuki and his colleagues in Japan in 1976 (Uchi- briefly. et al Oncogene (2005) 24, 5931–5937. doi:10.1038/sj.onc.1208981 yama ., 1977). The reports from his laboratory described unique characteristics of the leukemia such as Keywords: HTLV-1; transcription; tumor-suppressor unusual morphology of leukemic cells with lobulated genes; cell cycle; epidemiology nuclei and surface phenotypes. The most striking feature of the disease was the Kyushu (West part of Japan) origin of most of the patients who were identified in Kyoto where Takatsuki’s group performed their research. Takatsuki’s findings were later confirmed by extensive epidemiology and a clear clustering of Introduction ATL cases in the Kyushu area strongly suggested a unique etiology (Hinuma et al., 1981a). At the time, When virologists and oncologists are challenged to this novel discovery elicited enormous attention from identify human tumor viruses, they dream of the physicians, virologists, epidemiologists and oncologists, contribution of such viruses towards understanding but still there was no clue as to how to search for an cancers and answering questions such as how and why etiology. -

Viral Vectors
OCCUPATIONAL HEALTH CONSIDERATIONS FOR WORK WITH LENTIVIRAL VECTORS GARY R. FUJIMOTO, M.D. OCCUPATIONAL MEDICINE CONSULTANT OCTOBER 6, 2014 Disclosure: Lecture includes off-label use of antiretroviral medications VIRAL VECTORS Definition: Viruses engineered to deliver foreign genetic material (transgene) to cells Many viral vectors deliver the genetic material into the host cells but not into the host genome where the virus replicates (unless replication incompetent) Retroviral and lentiviral vectors deliver genetic transgenes into the host chromosomes LENTIVIRAL VECTORS Human immunodeficiency virus (HIV) is a lentivirus that infects both dividing and non-dividing cells Use of the HIV virus as a viral vector has required the reengineering of the virus to achieve safe gene transfer Since HIV normally targets CD4 cells, replacing the HIV envelope gene with vesicular stomatitis virus glycoprotein (VSV-G) expands the infectious range of the vector and modes of transmission LENTIVIRAL VECTORS Remember: replication deficient lentiviral vectors integrate the vector into the host chromosomes 3rd and 4th generation constructs unlikely to become replication competent with enhanced safety due to self- inactivating vectors (however, consider present or future HIV infection) Replication deficient lentiviral vectors should be regarded as single-event infectious agents Many researchers regard these agents as relatively benign although transgene integration does occur with generally unknown effects LENTIVIRAL OCCUPATIONAL EXPOSURES Lentiviral -

SIVISV.BOOK Layout 1
SEDE Piattaforma FAD Nadirex http://nadirex.dnaconnect.sm ORGANIZING SECRETARIAT AND PROVIDER NR. 265 Nadirex International S.r.l. Via Riviera, 39 - 27100 Pavia Tel. +39.0382.525714 Fax. +39.0382.525736 Contact: Gloria Molla [email protected] mob. +39 347 8589333 Contact: Francesca Granata [email protected] www.nadirex.com www.congressosivsiv.com ORGANIZING COMMITTEE PRESIDENT Arnaldo Caruso (Brescia, Italy) CHAIRS Guido Antonelli (Rome, Italy) Franco Buonaguro (Naples, Italy) Arnaldo Caruso (Brescia, Italy) Massimiliano Galdiero (Naples, Italy) Giuseppe Portella (Naples, Italy) SCIENTIFIC SECRETARIAT Francesca Caccuri (Brescia, Italy) Rossana Cavallo (Turin, Italy) Massimo Clementi (Milan, Italy) Gianluigi Franci (Salerno, Italy) Maria Cristina Parolin (Padua, Italy) Alessandra Pierangeli (Rome, Italy) Luisa Rubino (Bari, Italy) Gabriele Vaccari (Rome, Italy) EXECUTIVE BOARD Guido Antonelli (Rome, Italy) Franco Buonaguro (Naples, Italy) Arnaldo Caruso (Brescia, Italy) Massimiliano Galdiero (Naples, Italy) Giuseppe Portella (Naples, Italy) 3 EXECUTIVE BOARD PRESIDENT Arnaldo Caruso (Brescia, Italy) VICE PRESIDENT Canio Buonavoglia, University of Bari (Bari, Italy) SECRETARY Giorgio Gribaudo, University of Turin (Turin, Italy) TREASURER Luisa Rubino, National Research Council (Bari, Italy) ADVISER Guido Antonelli, University of Rome “La Sapienza” (Rome, Italy) ADVISORY COUNCIL Elisabetta Affabris (Rome, Italy) Fausto Baldanti (Pavia, Italy) Lawrence Banks (Trieste, Italy) Roberto Burioni (Milan, Italy) Arianna Calistri -

Viral Vectors
505 Oldham Court Lexington, KY 40502 Phone: (859) 257-1049 Fax: (859) 323-3838 E-Mail: [email protected] http://ehs.uky.edu/biosafety VIRAL VECTORS WHAT IS A VIRAL VECTOR? Viral vectors work like a “nanosyringe” to deliver nucleic acid to a target. They are often more efficient than other transfection methods, are useful for whole organism studies, have a relatively low toxicity, and are a likely route for human gene transfer. All viral vectors require a host for replication. The production of a viral vector is typically separated from the ability of the viral vector to infect cells. While viral vectors are not typically considered infectious agents, they do maintain their ability to “infect” cells. Viral vectors just don’t replicate (although there are some replicating viral vectors in use) under experimental conditions. An HIV- based lentiviral vector no longer possesses the ability to infect an individual with HIV, but it does maintain the ability to enter a cell and express genetic information. This is why viral vectors are useful, but also require caution. If a viral vector can transfect a human cell line on a plate, it can also transfect YOUR cells if accidentally exposed. SAFETY CONSIDERATIONS FOR ALL VIRAL VECTORS When utilizing ANY viral vector, the following questions must be addressed… 1. What potential does your method of viral vector production have to generate a replication competent virus? a. Generation of viral vector refers to the number of recombination events required to form a replication competent virus. For example, if you’re using a lentivirus that is split up between 4 plasmids (gag/pol, VSV-g, rev, transgene), 3 recombination events must take place to create a replication competent virus, therefore you are using a 3rd generation lentiviral vector. -

RNA Viruses As Tools in Gene Therapy and Vaccine Development
G C A T T A C G G C A T genes Review RNA Viruses as Tools in Gene Therapy and Vaccine Development Kenneth Lundstrom PanTherapeutics, Rte de Lavaux 49, CH1095 Lutry, Switzerland; [email protected]; Tel.: +41-79-776-6351 Received: 31 January 2019; Accepted: 21 February 2019; Published: 1 March 2019 Abstract: RNA viruses have been subjected to substantial engineering efforts to support gene therapy applications and vaccine development. Typically, retroviruses, lentiviruses, alphaviruses, flaviviruses rhabdoviruses, measles viruses, Newcastle disease viruses, and picornaviruses have been employed as expression vectors for treatment of various diseases including different types of cancers, hemophilia, and infectious diseases. Moreover, vaccination with viral vectors has evaluated immunogenicity against infectious agents and protection against challenges with pathogenic organisms. Several preclinical studies in animal models have confirmed both immune responses and protection against lethal challenges. Similarly, administration of RNA viral vectors in animals implanted with tumor xenografts resulted in tumor regression and prolonged survival, and in some cases complete tumor clearance. Based on preclinical results, clinical trials have been conducted to establish the safety of RNA virus delivery. Moreover, stem cell-based lentiviral therapy provided life-long production of factor VIII potentially generating a cure for hemophilia A. Several clinical trials on cancer patients have generated anti-tumor activity, prolonged survival, and