Gene Therapies Push Viral Vector Production
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mobile Genetic Elements in Streptococci
Curr. Issues Mol. Biol. (2019) 32: 123-166. DOI: https://dx.doi.org/10.21775/cimb.032.123 Mobile Genetic Elements in Streptococci Miao Lu#, Tao Gong#, Anqi Zhang, Boyu Tang, Jiamin Chen, Zhong Zhang, Yuqing Li*, Xuedong Zhou* State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu, PR China. #Miao Lu and Tao Gong contributed equally to this work. *Address correspondence to: [email protected], [email protected] Abstract Streptococci are a group of Gram-positive bacteria belonging to the family Streptococcaceae, which are responsible of multiple diseases. Some of these species can cause invasive infection that may result in life-threatening illness. Moreover, antibiotic-resistant bacteria are considerably increasing, thus imposing a global consideration. One of the main causes of this resistance is the horizontal gene transfer (HGT), associated to gene transfer agents including transposons, integrons, plasmids and bacteriophages. These agents, which are called mobile genetic elements (MGEs), encode proteins able to mediate DNA movements. This review briefly describes MGEs in streptococci, focusing on their structure and properties related to HGT and antibiotic resistance. caister.com/cimb 123 Curr. Issues Mol. Biol. (2019) Vol. 32 Mobile Genetic Elements Lu et al Introduction Streptococci are a group of Gram-positive bacteria widely distributed across human and animals. Unlike the Staphylococcus species, streptococci are catalase negative and are subclassified into the three subspecies alpha, beta and gamma according to the partial, complete or absent hemolysis induced, respectively. The beta hemolytic streptococci species are further classified by the cell wall carbohydrate composition (Lancefield, 1933) and according to human diseases in Lancefield groups A, B, C and G. -

Gutless Adenovirus: Last-Generation Adenovirus for Gene Therapy
Gene Therapy (2005) 12, S18–S27 & 2005 Nature Publishing Group All rights reserved 0969-7128/05 $30.00 www.nature.com/gt CONFERENCE PAPER Gutless adenovirus: last-generation adenovirus for gene therapy R Alba1, A Bosch1 and M Chillon1,2 1Gene Therapy Laboratory, Department of Biochemistry and Molecular Biology, Center of Animal Biotechnology and Gene Therapy (CBATEG), Universitat Auto`noma de Barcelona, Bellaterra, Spain; and 2Institut Catala` de Recerca i Estudis Avanc¸ats (ICREA), Barcelona, Spain Last-generation adenovirus vectors, also called helper-depen- viral coding regions, gutless vectors require viral proteins dent or gutless adenovirus, are very attractive for gene therapy supplied in trans by a helper virus. To remove contamination because the associated in vivo immune response is highly by a helper virus from the final preparation, different systems reduced compared to first- and second-generation adenovirus based on the excision of the helper-packaging signal have vectors, while maintaining high transduction efficiency and been generated. Among them, Cre-loxP system is mostly tropism. Nowadays, gutless adenovirus is administered in used, although contamination levels still are 0.1–1% too high different organs, such as the liver, muscle or the central to be used in clinical trials. Recently developed strategies to nervous system achieving high-level and long-term transgene avoid/reduce helper contamination were reviewed. expression in rodents and primates. However, as devoid of all Gene Therapy (2005) 12, S18–S27. doi:10.1038/sj.gt.3302612 Keywords: adenovirus; gutless; helper-dependent vectors; in vivo gene therapy Introduction clinical for more information). Nowadays, adenovirus vectors are applied to treat cancer, monogenic disorders, Gene therapy for most genetic diseases requires expres- vascular diseases and others complications. -

Viral Vectors and Biological Safety
Viral Vectors and Biological Safety Viral vectors are often designed so that they can enter human cells and deliver genes of interest. Viral vectors are usually replication-deficient – genes necessary for replication of the virus are removed from the vector and supplied separately through plasmids, helper virus, or packaging cell lines. There are several biosafety concerns that arise with the use of viral vectors including: 1) Tropism (host range) – viral vectors that can enter (infect) human cells are often used. 2) Replication-deficient viral vectors can gain back the deleted genes required for replication (become replication-competent) through recombination – referred to as replication-competent virus (RCV) breakthroughs. 3) Genes may be expressed in tissues and/or organisms where they are normally not expressed. In the case of some genes such as oncogenes, this could have far-reaching negative consequences. When evaluating safety for use of viral vectors, a number of factors need to be considered including: Risk Group (RG) of the organism; tropism (organism and tissue); route of transmission; whether the virus integrates into the host genome; and the specific gene(s) being introduced. Please contact the Office of Biological Safety (OBS) for more information on physical barriers and safety practices to use with specific viral vectors. This article concentrates on biological barriers that can be employed to improve safety when using viral vectors. Viral vectors frequently used are: • Retrovirus/lentivirus • Adenovirus • Adeno-associated virus (AAV) • Poxvirus • Herpes virus • Alphavirus • Baculovirus Amphotropic murine leukemia virus (MLV) – also called Moloney murine leukemia virus (MMLV) – and adenovirus are common viral vectors used to introduce genes into human cells. -
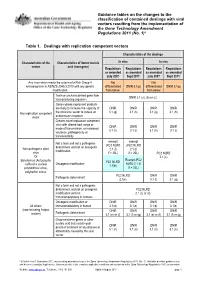
Guidance Tables on the Changes To
Guidance tables on the changes to the classification of contained dealings with viral vectors resulting from the implementation of the Gene Technology Amendment Regulations 2011 (No. 1)* Table 1. Dealings with replication competent vectors Characteristics of the dealings Characteristics of the Characteristics of donor nucleic In vitro In vivo vector acid (transgene) Regulations Regulations Regulations Regulations as amended as amended as amended as amended July 2007 Sept 2011* July 2007 Sept 2011* Any virus which meets the criteria of a Risk Group 4 Not Not microorganism in AS/NZS 2243.3:2010 with any genetic differentiated DNIR 3.1(p) differentiated DNIR 3.1(p) modification from below from below Toxin or uncharacterised gene from DNIR 3.1 (a), (b) or (c) toxin producing organism Genes whose expressed products are likely to increase the capacity of DNIR DNIR DNIR DNIR Any replication competent the virus/viral vector to induce an 3.1 (g) 3.1 (h) 3.1 (g) 3.1 (h) vector autoimmune response Creates novel replication competent virus with altered host range or DNIR DNIR DNIR DNIR mode of transmission, or increased 3.1 (h) 3.1 (i) 3.1 (h) 3.1 (i) virulence, pathogenicity or transmissibility exempt exempt Not a toxin and not a pathogenic (PC2 NLRD (PC2 NLRD determinant and not an oncogenic Non-pathogenic plant 2.1 (f) 2.1 (f) modification virus if > 25L) if > 25L) PC2 NLRD Or 2.1 (c) Exempt (PC2 Baculovirus (Autographa PC1 NLRD Oncogenic modification NLRD 2.1 (f) californica nuclear 1.1(b) polyhedrosis virus), if > 25L) polyhedrin minus PC2 NLRD -

Congressional Record United States Th of America PROCEEDINGS and DEBATES of the 116 CONGRESS, SECOND SESSION
E PL UR UM IB N U U S Congressional Record United States th of America PROCEEDINGS AND DEBATES OF THE 116 CONGRESS, SECOND SESSION Vol. 166 WASHINGTON, THURSDAY, DECEMBER 3, 2020 No. 204 House of Representatives The House met at 10 a.m. and was These are the people who walked in Doug Hartman, Karen Hasara, Holly called to order by the Speaker pro tem- parades; they helped pass out balloons, Healey, Brian Heckert, Bob pore (Mr. CUELLAR). candy, and political literature; they Hermsmeyer, Dennis Herrington, Nita f carried signs; they put up and took Hill, Mark and Elaine Hoffman, Nancy down political signs of all sizes; they Kimme, Bob Kjellander, Gwen Klinger, DESIGNATION OF SPEAKER PRO helped stuff mail and phone-bank; they Doug Knebel, Lynn Koch, Gale and Pat TEMPORE organized fundraisers, both big and Koelling, Greg Knott, J.C. Kowa, Kel- The SPEAKER pro tempore laid be- small; they manned booths at county vin Kuneth, Keith and Judy Loemker, fore the House the following commu- fairs. Kay Long, Tom and Robin Long, Sen- nication from the Speaker: What causes people to give up their ator David Luechtefeld, Curt and Lu WASHINGTON, DC, time, their talents and possessions to a Maddox, Tony Marsh, Mark and Carol December 3, 2020. candidate, party, or cause? It is at the Mestemacher, Don and Joanne Metzler, I hereby appoint the Honorable HENRY heart of a representative democracy, Guy Michael, Tom and Robin Long. CUELLAR to act as Speaker pro tempore on our constitutional Republic. Kathy Lynch, Kathy Lydon, Andy this day. -

Health Services COVID-19 Situation Report 01.15.2021
Dear Community Members, We welcome 2021 with the arrival of vaccines in the fight to defeat COVID-19. Just as we have been pushing for adoption of the precautions we all know work to include masking, hand hygiene and physical distancing, we must also push for high rates of vaccination within our communities if we hope to overcome this virus. This will require trust in the COVID vaccination process, from the development, distribution and administration of a safe and effective vaccine as well as willingness to get vaccinated. Our hope is simple, we urge you to get the COVID-19 vaccine and share your experience with others. Several new variants of the virus causing COVID-19 have been reported, including one in the United Kingdom (UK), one in South Africa and one from Brazil. As of 1/10/2021, cases of the UK variant have been confirmed in multiple regions of the United States and Canada. None have yet been found in Alaska. CDC is monitoring the new variants and encouraging states to do more sequencing (sampling positive specimens to identify variant). The Alaska State Public Health Laboratories routinely sequence a subset of positive tests including all that contain the pattern associated with the UK strain. These variants seem to spread more easily and quickly than other variants, which may lead to more cases of COVID-19. Currently, there is no evidence that these variants cause more severe illness or increased risk of death. However, an increase in the number of cases will put more strain on health care resources, lead to more hospitalizations, and potentially more deaths. -

Viral Vectors
OCCUPATIONAL HEALTH CONSIDERATIONS FOR WORK WITH LENTIVIRAL VECTORS GARY R. FUJIMOTO, M.D. OCCUPATIONAL MEDICINE CONSULTANT OCTOBER 6, 2014 Disclosure: Lecture includes off-label use of antiretroviral medications VIRAL VECTORS Definition: Viruses engineered to deliver foreign genetic material (transgene) to cells Many viral vectors deliver the genetic material into the host cells but not into the host genome where the virus replicates (unless replication incompetent) Retroviral and lentiviral vectors deliver genetic transgenes into the host chromosomes LENTIVIRAL VECTORS Human immunodeficiency virus (HIV) is a lentivirus that infects both dividing and non-dividing cells Use of the HIV virus as a viral vector has required the reengineering of the virus to achieve safe gene transfer Since HIV normally targets CD4 cells, replacing the HIV envelope gene with vesicular stomatitis virus glycoprotein (VSV-G) expands the infectious range of the vector and modes of transmission LENTIVIRAL VECTORS Remember: replication deficient lentiviral vectors integrate the vector into the host chromosomes 3rd and 4th generation constructs unlikely to become replication competent with enhanced safety due to self- inactivating vectors (however, consider present or future HIV infection) Replication deficient lentiviral vectors should be regarded as single-event infectious agents Many researchers regard these agents as relatively benign although transgene integration does occur with generally unknown effects LENTIVIRAL OCCUPATIONAL EXPOSURES Lentiviral -

BIOPRODUCTION INTRODUCTION Mabdesign’S Immunowatch Is a One-Of-A-Kind Information Monitoring Newsletter in the Field of Biologics
IMMUNOWATCH Edition n°3 - July 2021 BIOPRODUCTION INTRODUCTION MabDesign’s Immunowatch is a one-of-a-kind information monitoring newsletter in the field of biologics. Its aim is to provide members of our association with the most recent and pertinent data gathered or generated through the key expertise of MabDesign and its collaborators in scientific research, business intelligence, market analysis and intellectual property. It’s general format includes a market study research, financial and economic data, invited contributions from scientists working in the industry or in academia and a section dedicated to intellectual property. The content of each edition is decided by an editorial composed of two field experts. While each edition usually focuses on one trending type of biologics, this current issue has been adapted to cover the bioprocessing aspects of these products and serve as a general introduction to subject. This editorial choice has been motivated by recent development, in terms of innovation and national strategies and by MabDesign’s ongoing and/or upcoming actions and events in the bioprocessing field. Immunowatch is done in collaboration with the MAbMapping Unit of the Ambition Recherche & Développement (ARD) Biomédicaments 2020 Phase II programme, funded by the Centre Val de Loire region. 2 Table of content 4. Editorial 7. Global BioPharmaceutical production market 8. Biopharmaceuticals to produce in the future 9. Bioprocessing 10. Biopharmaceuticals CDMO market 12. Bioprocessing companies in France 14. Bioprocessing companies in France: Focus COVID-19 16. Special articleS 17. Biologics and their chemical counterparts 19. Human and animal cells as factories for therapeutic molecules 29. Production processes for biotherapeutics 37. -

Defense Production Act
United States Government Accountability Office Report to Congressional Committees November 2020 DEFENSE PRODUCTION ACT Opportunities Exist to Increase Transparency and Identify Future Actions to Mitigate Medical Supply Chain Issues GAO-21-108 November 2020 DEFENSE PRODUCTION ACT Opportunities Exist to Increase Transparency and Identify Future Actions to Mitigate Medical Supply Highlights of GAO-21-108, a report to Chain Issues congressional committees Why GAO Did This Study What GAO Found COVID-19 has put the U.S. health care Federal agencies used the Defense Production Act (DPA) to help address system under severe strain, including medical supply shortages from COVID-19. The DPA gives agencies the authority affecting the federal government’s to (1) prioritize contracts for medical supplies so those orders get preference over ability to buy and maintain critical others, and (2) expand domestic production of medical supplies. GAO identified medical supplies to treat patients and 43 contracts and agreements—initially valued at about $3.9 billion—where protect health care workers. agencies placed priority ratings on or funded domestic production expansion In March 2020 agencies began using projects for COVID-19 medical supplies (see figure). Department of Health and DPA authorities to rapidly obtain and Human Services (HHS) and Federal Emergency Management Agency (FEMA) expand domestic production of medical officials stated that nearly all the approximately 181,000 ventilators and 166.5 supplies for COVID-19. The CARES million of the respirators they placed on priority rated contracts, respectively, Act provided the Department of have been delivered as of September 2020. Defense (DOD) $1 billion for DPA purchases related to COVID-19. -

Table of Contents Introduction
Congressional and Federal Agency Responses and Opportunities Regarding the COVID-19 Outbreak Lewis-Burke Associates LLC July 1, 2020 Table of Contents Introduction .................................................................................................................................. 4 Congressional and Federal Updates .............................................................................................. 5 Federal Guidance Related to Research and Higher Education ...................................................... 7 Office of Management and Budget (OMB) .................................................................................... 7 Department of Education (ED) ....................................................................................................... 7 Update: National Institutes of Health (NIH) ................................................................................. 11 Department of Health and Human Services (HHS) ...................................................................... 12 Administration for Community Living (ACL)................................................................................. 13 Centers for Medicare and Medicaid Services (CMS) ................................................................... 14 Update: Food and Drug Administration (FDA) ............................................................................. 15 Update: Centers for Disease Control and Prevention (CDC) ....................................................... 16 Agency for Healthcare -

ODP COVID-19 Vaccination Update
ODP COVID-19 Vaccination Update February 2, 2021 1 As of 2/1/2021 World Cases: 103,244,127 World Deaths: 2,233,610 United States Cases: 26,231,939 United States Deaths: 442,399 2 02/01/2021 3 Date of Data Extraction 1/27/2021 4 Statewide Confirmed Individual and Staff COVID-19 Cases 500 450 400 350 300 250 200 150 100 50 0 4/29/2020 5/29/2020 6/29/2020 7/29/2020 8/29/2020 9/29/2020 2/2/21 10/29/2020 11/29/2020 12/29/2020 5 Individuals Staff COVID-19 Interim Vaccination Plan Prioritization Version: Jan 19, 2021 Major Updates: • As recommended by Operation Warp Speed on 1/12/21 adds individuals ages 65 and older and • Individuals ages 16-64 with certain underlying medical conditions in Phase 1A. • Updated list oF underlying conditions in- line with CDC (now includes Down Syndrome and Sickle Cell Disease) • Added in 1A Healthcare Personnel “individuals providing services For the elderly and persons with disabilities including unpaid caregivers” 2/2/21 6 COVID-19 Interim Vaccination Plan Prioritization Version: Jan 19, 2021 Phase 1A • Long Term Care Facilities: Residents and staff of community group homes and ICFs • Healthcare Personnel: All DSPs supporting individuals through face-to-face service provision outside of an ICF or 6400 setting are included in Phase 1A; • Healthcare Personnel: individuals providing services for the elderly and persons with disabilities including unpaid caregivers Phase 1B • Individuals receiving home and community-based services – Office of Developmental Programs Home and Community-Based Services 2/2/21 7 Phase -

Will Plant-Based Vaccines Be the Answer?
Review Combating Human Viral Diseases: Will Plant-Based Vaccines Be the Answer? Srividhya Venkataraman 1,*, Kathleen Hefferon 1, Abdullah Makhzoum 2 and Mounir Abouhaidar 1 1 Virology Laboratory, Department of Cell & Systems Biology, University of Toronto, Toronto, ON M5S 3B2, Canada; [email protected] (K.H.); [email protected] (M.A.) 2 Department of Biological Sciences & Biotechnology, Botswana International University of Science & Technology, Palapye, Botswana; [email protected] * Correspondence: [email protected] Abstract: Molecular pharming or the technology of application of plants and plant cell culture to manufacture high-value recombinant proteins has progressed a long way over the last three decades. Whether generated in transgenic plants by stable expression or in plant virus-based transient ex- pression systems, biopharmaceuticals have been produced to combat several human viral diseases that have impacted the world in pandemic proportions. Plants have been variously employed in expressing a host of viral antigens as well as monoclonal antibodies. Many of these biopharmaceuti- cals have shown great promise in animal models and several of them have performed successfully in clinical trials. The current review elaborates the strategies and successes achieved in generating plant-derived vaccines to target several virus-induced health concerns including highly commu- nicable infectious viral diseases. Importantly, plant-made biopharmaceuticals against hepatitis B virus (HBV), hepatitis C virus (HCV), the cancer-causing virus human papillomavirus (HPV), human Citation: Venkataraman, S.; immunodeficiency virus (HIV), influenza virus, zika virus, and the emerging respiratory virus, Hefferon, K.; Makhzoum, A.; Abouhaidar, M. Combating Human severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) have been discussed.