This Training Module Is Required for All Personnel Listed on an IBC Protocol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mobile Genetic Elements in Streptococci
Curr. Issues Mol. Biol. (2019) 32: 123-166. DOI: https://dx.doi.org/10.21775/cimb.032.123 Mobile Genetic Elements in Streptococci Miao Lu#, Tao Gong#, Anqi Zhang, Boyu Tang, Jiamin Chen, Zhong Zhang, Yuqing Li*, Xuedong Zhou* State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu, PR China. #Miao Lu and Tao Gong contributed equally to this work. *Address correspondence to: [email protected], [email protected] Abstract Streptococci are a group of Gram-positive bacteria belonging to the family Streptococcaceae, which are responsible of multiple diseases. Some of these species can cause invasive infection that may result in life-threatening illness. Moreover, antibiotic-resistant bacteria are considerably increasing, thus imposing a global consideration. One of the main causes of this resistance is the horizontal gene transfer (HGT), associated to gene transfer agents including transposons, integrons, plasmids and bacteriophages. These agents, which are called mobile genetic elements (MGEs), encode proteins able to mediate DNA movements. This review briefly describes MGEs in streptococci, focusing on their structure and properties related to HGT and antibiotic resistance. caister.com/cimb 123 Curr. Issues Mol. Biol. (2019) Vol. 32 Mobile Genetic Elements Lu et al Introduction Streptococci are a group of Gram-positive bacteria widely distributed across human and animals. Unlike the Staphylococcus species, streptococci are catalase negative and are subclassified into the three subspecies alpha, beta and gamma according to the partial, complete or absent hemolysis induced, respectively. The beta hemolytic streptococci species are further classified by the cell wall carbohydrate composition (Lancefield, 1933) and according to human diseases in Lancefield groups A, B, C and G. -

Chapitre Quatre La Spécificité D'hôtes Des Virophages Sputnik
AIX-MARSEILLE UNIVERSITE FACULTE DE MEDECINE DE MARSEILLE ECOLE DOCTORALE DES SCIENCES DE LA VIE ET DE LA SANTE THESE DE DOCTORAT Présentée par Morgan GAÏA Né le 24 Octobre 1987 à Aubagne, France Pour obtenir le grade de DOCTEUR de l’UNIVERSITE AIX -MARSEILLE SPECIALITE : Pathologie Humaine, Maladies Infectieuses Les virophages de Mimiviridae The Mimiviridae virophages Présentée et publiquement soutenue devant la FACULTE DE MEDECINE de MARSEILLE le 10 décembre 2013 Membres du jury de la thèse : Pr. Bernard La Scola Directeur de thèse Pr. Jean -Marc Rolain Président du jury Pr. Bruno Pozzetto Rapporteur Dr. Hervé Lecoq Rapporteur Faculté de Médecine, 13385 Marseille Cedex 05, France URMITE, UM63, CNRS 7278, IRD 198, Inserm 1095 Directeur : Pr. Didier RAOULT Avant-propos Le format de présentation de cette thèse correspond à une recommandation de la spécialité Maladies Infectieuses et Microbiologie, à l’intérieur du Master des Sciences de la Vie et de la Santé qui dépend de l’Ecole Doctorale des Sciences de la Vie de Marseille. Le candidat est amené à respecter des règles qui lui sont imposées et qui comportent un format de thèse utilisé dans le Nord de l’Europe permettant un meilleur rangement que les thèses traditionnelles. Par ailleurs, la partie introduction et bibliographie est remplacée par une revue envoyée dans un journal afin de permettre une évaluation extérieure de la qualité de la revue et de permettre à l’étudiant de commencer le plus tôt possible une bibliographie exhaustive sur le domaine de cette thèse. Par ailleurs, la thèse est présentée sur article publié, accepté ou soumis associé d’un bref commentaire donnant le sens général du travail. -

Gutless Adenovirus: Last-Generation Adenovirus for Gene Therapy
Gene Therapy (2005) 12, S18–S27 & 2005 Nature Publishing Group All rights reserved 0969-7128/05 $30.00 www.nature.com/gt CONFERENCE PAPER Gutless adenovirus: last-generation adenovirus for gene therapy R Alba1, A Bosch1 and M Chillon1,2 1Gene Therapy Laboratory, Department of Biochemistry and Molecular Biology, Center of Animal Biotechnology and Gene Therapy (CBATEG), Universitat Auto`noma de Barcelona, Bellaterra, Spain; and 2Institut Catala` de Recerca i Estudis Avanc¸ats (ICREA), Barcelona, Spain Last-generation adenovirus vectors, also called helper-depen- viral coding regions, gutless vectors require viral proteins dent or gutless adenovirus, are very attractive for gene therapy supplied in trans by a helper virus. To remove contamination because the associated in vivo immune response is highly by a helper virus from the final preparation, different systems reduced compared to first- and second-generation adenovirus based on the excision of the helper-packaging signal have vectors, while maintaining high transduction efficiency and been generated. Among them, Cre-loxP system is mostly tropism. Nowadays, gutless adenovirus is administered in used, although contamination levels still are 0.1–1% too high different organs, such as the liver, muscle or the central to be used in clinical trials. Recently developed strategies to nervous system achieving high-level and long-term transgene avoid/reduce helper contamination were reviewed. expression in rodents and primates. However, as devoid of all Gene Therapy (2005) 12, S18–S27. doi:10.1038/sj.gt.3302612 Keywords: adenovirus; gutless; helper-dependent vectors; in vivo gene therapy Introduction clinical for more information). Nowadays, adenovirus vectors are applied to treat cancer, monogenic disorders, Gene therapy for most genetic diseases requires expres- vascular diseases and others complications. -

Viral Vectors and Biological Safety
Viral Vectors and Biological Safety Viral vectors are often designed so that they can enter human cells and deliver genes of interest. Viral vectors are usually replication-deficient – genes necessary for replication of the virus are removed from the vector and supplied separately through plasmids, helper virus, or packaging cell lines. There are several biosafety concerns that arise with the use of viral vectors including: 1) Tropism (host range) – viral vectors that can enter (infect) human cells are often used. 2) Replication-deficient viral vectors can gain back the deleted genes required for replication (become replication-competent) through recombination – referred to as replication-competent virus (RCV) breakthroughs. 3) Genes may be expressed in tissues and/or organisms where they are normally not expressed. In the case of some genes such as oncogenes, this could have far-reaching negative consequences. When evaluating safety for use of viral vectors, a number of factors need to be considered including: Risk Group (RG) of the organism; tropism (organism and tissue); route of transmission; whether the virus integrates into the host genome; and the specific gene(s) being introduced. Please contact the Office of Biological Safety (OBS) for more information on physical barriers and safety practices to use with specific viral vectors. This article concentrates on biological barriers that can be employed to improve safety when using viral vectors. Viral vectors frequently used are: • Retrovirus/lentivirus • Adenovirus • Adeno-associated virus (AAV) • Poxvirus • Herpes virus • Alphavirus • Baculovirus Amphotropic murine leukemia virus (MLV) – also called Moloney murine leukemia virus (MMLV) – and adenovirus are common viral vectors used to introduce genes into human cells. -

Replication-Defective Chimeric Helper Proviruses and Factors Affecting Generation of Competent Virus
MOLECULAR AND CELLULAR BIOLOGY, May 1987, p. 1797-1806 Vol. 7, No. 5 0270-7306/87/051797-10$02.00/0 Copyright © 1987, American Society for Microbiology Replication-Defective Chimeric Helper Proviruses and Factors Affecting Generation of Competent Virus: Expression of Moloney Murine Leukemia Virus Structural Genes via the Metallothionein Promoter ROBERT A. BOSSELMAN,* ROU-YIN HSU, JOAN BRUSZEWSKI, SYLVIA HU, FRANK MARTIN, AND MARGERY NICOLSON Amgen, Thousand Oaks, California 91320 Received 15 October 1986/Accepted 28 January 1987 Two chimeric helper proviruses were derived from the provirus of the ecotropic Moloney murine leukemia virus by replacing the 5' long terminal repeat and adjacent proviral sequences with the mouse metallothionein I promoter. One of these chimeric proviruses was designed to express the gag-pol genes of the virus, whereas the other was designed to express only the env gene. When transfected into NIH 3T3 cells, these helper proviruses failed to generate competent virus but did express Zn2 -inducible trans-acting viral functions needed to assemble infectious vectors. One helper cell line (clone 32) supported vector assembly at levels comparable to those supported by the Psi-2 and PA317 cell lines transfected with the same vector. Defective proviruses which carry the neomycin phosphotransferase gene and which lack overlapping sequence homology with the 5' end of the chimeric helper proviruses could be transfected into the helper cell line without generation of replication-competent virus. Mass cultures of transfected helper cells produced titers of about 104 G418r CFU/ml, whereas individual clones produced titers between 0 and 2.6 x 104 CFU/ml. -
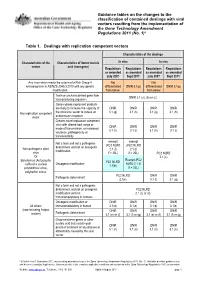
Guidance Tables on the Changes To
Guidance tables on the changes to the classification of contained dealings with viral vectors resulting from the implementation of the Gene Technology Amendment Regulations 2011 (No. 1)* Table 1. Dealings with replication competent vectors Characteristics of the dealings Characteristics of the Characteristics of donor nucleic In vitro In vivo vector acid (transgene) Regulations Regulations Regulations Regulations as amended as amended as amended as amended July 2007 Sept 2011* July 2007 Sept 2011* Any virus which meets the criteria of a Risk Group 4 Not Not microorganism in AS/NZS 2243.3:2010 with any genetic differentiated DNIR 3.1(p) differentiated DNIR 3.1(p) modification from below from below Toxin or uncharacterised gene from DNIR 3.1 (a), (b) or (c) toxin producing organism Genes whose expressed products are likely to increase the capacity of DNIR DNIR DNIR DNIR Any replication competent the virus/viral vector to induce an 3.1 (g) 3.1 (h) 3.1 (g) 3.1 (h) vector autoimmune response Creates novel replication competent virus with altered host range or DNIR DNIR DNIR DNIR mode of transmission, or increased 3.1 (h) 3.1 (i) 3.1 (h) 3.1 (i) virulence, pathogenicity or transmissibility exempt exempt Not a toxin and not a pathogenic (PC2 NLRD (PC2 NLRD determinant and not an oncogenic Non-pathogenic plant 2.1 (f) 2.1 (f) modification virus if > 25L) if > 25L) PC2 NLRD Or 2.1 (c) Exempt (PC2 Baculovirus (Autographa PC1 NLRD Oncogenic modification NLRD 2.1 (f) californica nuclear 1.1(b) polyhedrosis virus), if > 25L) polyhedrin minus PC2 NLRD -

Viral Envelope Are Controlled by the Helper Virus Used to Activate The
DETERMINING FACTOR IN THE CAPACITY OF ROUS SARCOMA VIRUS TO INDUCE TUMORS IN MAMMALS* By HIDESABURO HANAFUSA AND TERUKO HANAFUSA COLLMGE DE FRANCE, LABORATOIRE DE MIDECINE EXPARIMENTALE, PARIS Communidated by Ahdre Lwoff, January 24, 1966 Since Zilber and Kriukoval and Svet-Moldavsky' demonstrated that some strains of Rous sarcoma virus (RSV) are capable of inducing hemorrhagic cysts or sarcomas in newborn rats, numerous attempts have been made to induce tumors by RSVin various species of mammals.3-9 One of the remarkable aspects revealed by these investigations is the strain differences in RSV for this capacity.6-9 Certain strains, such as the Schmidt-Ruppin strain, are active, while others, such as the Bryan strain, are generally inactive in mammals. The cause of such strain differ- ences has not been elucidated. Our previous studies have shown that the Bryan high-titer strain of RSV is a defective virus which cannot reproduce without the help of any one of the avian leukosis viruses.'0 The defectiveness derives from the inability of this strain of RSV to induce the synthesis of its own viral envelope."I An essential role played by the helper virus is to provide the envelope to the RSV genome, whose replica- tion occurs in the infected cells without the aid of helper virus. Thus, several properties of RSV which presumably depend in some way on the character of the viral envelope are controlled by the helper virus used to activate the RSV.11-13 These properties include the antigenicity, the sensitivity to the interference induced by the helper virus, and the host range among genetically different chick embryos. -

Satellite RNA-Mediated Resistance to Turnip Crinkle Virus in Arabidopsis Lnvolves a Reduction in Virus Movement
The Plant Cell, Vol. 9, 2051-2063, November 1997 O 1997 American Society of Plant Physiologists Satellite RNA-Mediated Resistance to Turnip Crinkle Virus in Arabidopsis lnvolves a Reduction in Virus Movement Qingzhong Kong, Jianlong Wang, and Anne E. Simon’ Department of Biochemistry and Molecular Biology and Program in Molecular and Cellular Biology, University of Massachusetts, Amherst, Massachusetts O1 003-4505 Satellite RNAs (sat-RNAs) are parasites of viruses that can mediate resistance to the helper virus. We previously showed that a sat-RNA (sat-RNA C) of turnip crinkle virus (TCV), which normally intensifies symptoms of TCV, is able to attenuate symptoms when TCV contains the coat protein (CP) of cardamine chlorotic fleck virus (TCV-CPccw).We have now determined that sat-RNA C also attenuates symptoms of TCV containing an alteration in the initiating AUG of the CP open reading frame (TCV-CPm). TCV-CPm, which is able to move systemically in both the TCV-susceptible ecotype Columbia (Col-O) and the TCV-resistant ecotype Dijon (Di-O), produced a reduced level of CP and no detectable virions in infected plants. Sat-RNA C reduced the accumulation of TCV-CPm by <25% in protoplasts while reducing the level of TCV-CPm by 90 to 100% in uninoculated leaves of COLO and Di-O. Our results suggest that in the presence of a re- duced level of a possibly altered CP, sat-RNA C reduces virus long-distance movement in a manner that is independent of the salicylic acid-dependent defense pathway. INTRODUCTION Plants exhibit different types of resistance to plant viruses, on virus association for replication and spread in plants. -

Helper-Dependent Adenoviral Vectors
ndrom Sy es tic & e G n e e n G e f T o Rosewell, J Genet Syndr Gene Ther 2011, S:5 Journal of Genetic Syndromes h l e a r n a r p DOI: 10.4172/2157-7412.S5-001 u y o J & Gene Therapy ISSN: 2157-7412 Review Article Open Access Helper-Dependent Adenoviral Vectors Amanda Rosewell, Francesco Vetrini, and Philip Ng* Department of Molecular and Human Genetics, Baylor College of Medicine, Houston, TX, 77030 USA Abstract Helper-dependent adenoviral vectors are devoid of all viral coding sequences, possess a large cloning capacity, and can efficiently transduce a wide variety of cell types from various species independent of the cell cycle to mediate long-term transgene expression without chronic toxicity. These non-integrating vectors hold tremendous potential for a variety of gene transfer and gene therapy applications. Here, we review the production technologies, applications, obstacles to clinical translation and their potential resolutions, and the future challenges and unanswered questions regarding this promising gene transfer technology. Introduction DNA polymerase and terminal protein precursor (pTP). The E3 region, which is dispensable for virus growth in cell culture, encodes at least Helper-dependent adenoviral vectors (HDAd) are deleted of all seven proteins most of which are involved in host immune evasion. The viral coding sequences, can efficiently transduce a wide variety of cell E4 region encodes at least six proteins, some functioning to facilitate types from various species independent of the cell cycle, and can result in DNA replication, enhance late gene expression and decrease host long-term transgene expression. -

Viral Vectors
OCCUPATIONAL HEALTH CONSIDERATIONS FOR WORK WITH LENTIVIRAL VECTORS GARY R. FUJIMOTO, M.D. OCCUPATIONAL MEDICINE CONSULTANT OCTOBER 6, 2014 Disclosure: Lecture includes off-label use of antiretroviral medications VIRAL VECTORS Definition: Viruses engineered to deliver foreign genetic material (transgene) to cells Many viral vectors deliver the genetic material into the host cells but not into the host genome where the virus replicates (unless replication incompetent) Retroviral and lentiviral vectors deliver genetic transgenes into the host chromosomes LENTIVIRAL VECTORS Human immunodeficiency virus (HIV) is a lentivirus that infects both dividing and non-dividing cells Use of the HIV virus as a viral vector has required the reengineering of the virus to achieve safe gene transfer Since HIV normally targets CD4 cells, replacing the HIV envelope gene with vesicular stomatitis virus glycoprotein (VSV-G) expands the infectious range of the vector and modes of transmission LENTIVIRAL VECTORS Remember: replication deficient lentiviral vectors integrate the vector into the host chromosomes 3rd and 4th generation constructs unlikely to become replication competent with enhanced safety due to self- inactivating vectors (however, consider present or future HIV infection) Replication deficient lentiviral vectors should be regarded as single-event infectious agents Many researchers regard these agents as relatively benign although transgene integration does occur with generally unknown effects LENTIVIRAL OCCUPATIONAL EXPOSURES Lentiviral -

Helper Virus-Free, Optically Controllable, and Two-Plasmid-Based Production of Adeno-Associated Virus Vectors of Serotypes 1 to 6 Dirk Grimm,1 Mark A
doi:10.1016/S1525-0016(03)00095-9 METHOD Helper Virus-Free, Optically Controllable, and Two-Plasmid-Based Production of Adeno-associated Virus Vectors of Serotypes 1 to 6 Dirk Grimm,1 Mark A. Kay,1,* and Juergen A. Kleinschmidt2 1 Department of Pediatrics and Department of Genetics, Stanford University School of Medicine, 300 Pasteur Drive, Stanford, California 94305 2 Department of Applied Tumor Virology, German Cancer Research Center, Im Neuenheimer Feld 242, D-69121 Heidelberg, Germany *To whom correspondence and reprint requests should be addressed. Fax: (650) 498-6540. E-mail: [email protected]. We present a simple and safe strategy for producing high-titer adeno-associated virus (AAV) vectors derived from six different AAV serotypes (AAV-1 to AAV-6). The method, referred to as “HOT,” is helper virus free, optically controllable, and based on transfection of only two plasmids, i.e., an AAV vector construct and one of six novel AAV helper plasmids. The latter were engineered to carry AAV serotype rep and cap genes together with adenoviral helper functions, as well as unique fluorescent protein expression cassettes, allowing confirmation of successful transfection and identification of the transfected plasmid. Cross-packaging of vector DNA derived from AAV-2, -3, or -6 was up to 10-fold more efficient using our novel plasmids, compared to a conservative adenovirus-dependent method. We also identified a variety of useful antibodies, allowing detec- tion of Rep or VP proteins, or assembled capsids, of all six AAV serotypes. Finally, we describe unique cell tropisms and kinetics of transgene expression for AAV serotype vectors in primary or transformed cells from four different species. -

Viral Vectors
505 Oldham Court Lexington, KY 40502 Phone: (859) 257-1049 Fax: (859) 323-3838 E-Mail: [email protected] http://ehs.uky.edu/biosafety VIRAL VECTORS WHAT IS A VIRAL VECTOR? Viral vectors work like a “nanosyringe” to deliver nucleic acid to a target. They are often more efficient than other transfection methods, are useful for whole organism studies, have a relatively low toxicity, and are a likely route for human gene transfer. All viral vectors require a host for replication. The production of a viral vector is typically separated from the ability of the viral vector to infect cells. While viral vectors are not typically considered infectious agents, they do maintain their ability to “infect” cells. Viral vectors just don’t replicate (although there are some replicating viral vectors in use) under experimental conditions. An HIV- based lentiviral vector no longer possesses the ability to infect an individual with HIV, but it does maintain the ability to enter a cell and express genetic information. This is why viral vectors are useful, but also require caution. If a viral vector can transfect a human cell line on a plate, it can also transfect YOUR cells if accidentally exposed. SAFETY CONSIDERATIONS FOR ALL VIRAL VECTORS When utilizing ANY viral vector, the following questions must be addressed… 1. What potential does your method of viral vector production have to generate a replication competent virus? a. Generation of viral vector refers to the number of recombination events required to form a replication competent virus. For example, if you’re using a lentivirus that is split up between 4 plasmids (gag/pol, VSV-g, rev, transgene), 3 recombination events must take place to create a replication competent virus, therefore you are using a 3rd generation lentiviral vector.