Supporting Information © Wiley-VCH 2007 69451 Weinheim, Germany
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
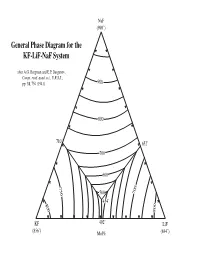
General Phase Diagram for the KF-Lif-Naf System
NaF (990˚) General Phase Diagram for the KF-LiF-NaF System after A.G. Bergman and E.P. Dergunov, Compt. rend. acad. sci., U.R.S.S., pp. 31, 754 (1941). 900 800 710˚ 652˚ 700 600 750 500 700 454˚ 800 800 KF 492˚ LiF (856˚) Mol% (844˚) Sodium Fluoride NaF 950˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 940˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 930˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 920˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 910˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 900˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 890˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 880˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 870˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 860˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 850˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 840˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 830˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 820˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 810˚ 810˚ ˚ KF 810 LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 800˚ 800˚ ˚ KF 800 LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 790˚ 790˚ ˚ KF 790 LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 780˚ 780˚ ˚ LiF KF 780 Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 770˚ 770˚ ˚ LiF KF 770 Potassium Fluoride -

United States Patent Office Patented July 1, 1969
3,453,337 United States Patent Office Patented July 1, 1969 1. 2 3,453,337 FLUORINATION OF HALOGENATED The presence in the reaction mixture of the two fluo ORGANIC COMPOUNDS rides, or the complex fluoride enables better yields of Royston Henry Bennett and David Walter Cottrell, Ayon highly fluorinated products to be obtained under less mouth, England, assignors to Imperial Smelting Cor severe reaction condions, markedly increases the amount poration (N.S.C.) Limited, London, England, a British of fluorination reagent reacted under otherwise similar company conditions and enables the fluorination reaction to be No brawing. Filed Feb. 19, 1965, Ser. No. 434,128 carried out (for the same yield of product) at a lower Claims priority, application Great Britain, Feb. 26, 1964, temperature with the consequent use of less costly ma 7,932/64 terials and techniques of reactor construction. The pres Int, C. C07c 25/04 10 ence of the fluorides enables the vapor phase reaction U.S. C. 260-650 2 Claims to be carried out (for the same yields) at lower pressure This invention relates to the fluorination of organic than the pressure involved in the reactions using only halogen compounds and more especially to a process the alkali metal fluorides as proposed hitherto. The fur for the production of highly fluorinated aromatic com ther possibility of using a continuous flow apparatus such pounds by the replacement of higher halogen atoms in 15 as a fluidised reactor will be apparent to those familiar halogeno-aromatic compounds by fluorine atoms. with the art. The presence of the two fluorides or com Aromatic halogenocarbons containing carbon and halo plex fluoride enables a lower temperature to be em gen atoms only can be reacted with alkali fluorides ployed than was hitherto believed to be necessary, with under various conditions to give yields of halofluoro a consequent reduction in the extent of thermal degrada aromatic compounds. -

Reaction of Potassium Fluoride with Organic Halogen Compounds. I
Reaction of Potassium Fluoride with Organic Halogen Compounds. I) Reactions of Potassium Fluoride with Organic Halides, Acids, aad Esters in presence ef Dimethyl Formamide and their Pyrolytic Decaboxylation in presence of Potassium Fluoride By You Sun Kim Atomic Energy Research Institute, Korea 有機 할로겐 化合物과 弗化加里의 反應 (第1報) 有機 할라어드, 酸 및 에스테르와 弗化加里의 디메칠 호쁨아마이드 溶蝶系 反應 및 高混■■脫炭酸-熱分解反應 金 裕 *善 (1963. 6. 19 受理) Abstract Reactions between potassium fluride with organic halogen-containning carboxylic acids in dimethyl formamide solvent gave a decarboxylation reaction for the case of fluoro carboxylic acids of the type of CF3 COOH, C3F7COOH, and C2F5COOH, whereas an additional partial fluorination together with dimeri zation reaction occured for the chlorine containning acids of the type of CH2CICOOH, CH3CHCICOOH, CHCI2COOH and o-Cl-CeHi-COOH. The phenyl halides showed no reactivity, but the halides with two electron attracting substituents on the benzene ring gave mainly dimerization reaction. The esters and alcohols gave an usual fluorination reaction. The same reactions in absence of the solvent at the elevated temperature increase the yield of the dimerized product and gave the cyclized product, fluorenone, in case of o-chlorobenzoic acid. It was found that the fluorination usually precede the decarboxylation reaction by checking the stiochemical sequence of reaction. Catalytic influence of potassium fluoride were discussed and the mechanism of the reaction was considered. 耍 約 「디메望호름아마이드」溶媒系에서 有機含할로겐化合物을 弗化加里와 反應시켜 본 結果 CFsCOOH, CsF’COOH, CzFQOOH 와 같은 含弗素有機酸에서는 脫炭酸反應이 일어나며, 含鹽素有機酸, CH2C1COOH. CH3CHC1COOH, CHC12- COOH 및 o-CK사LCOOH 은 一部 弗化反應이 일어 나고 雙合어imerization) 反應이 隨伴된다는 것을 究明하였다. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Effects of Potassium and Sodium Fluoride in Different
Dental Materials Journal 2020; : – Effects of potassium and sodium fluoride in different concentrations on micro- shear bond strength and inhibition of demineralization Ali AL-QAHTANI1, Go INOUE1, Ahmed ABDOU1, Toru NIKAIDO2 and Junji TAGAMI1 1 Department of Cariology and Operative Dentistry, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University (TMDU), 1-5-45 Yushima, Bunkyo-ku, Tokyo, Japan 2 Department of Operative Dentistry, Division of Oral Functional Science and Rehabilitation, School of Dentistry, Asahi University, Hozumi 1851, Mizuho, Gifu 501-0296, Japan Corresponding author, Go INOUE; E-mail: [email protected] The aim of this study was to investigate the effects of potassium fluoride (KF) and sodium fluoride (NaF) in different concentrations on micro-shear bond strength (µSBS) and their protective effects against acid. The enamel blocks were treated with several concentrations of KF and NaF. For µSBS, Clearfil SE Bond 2 was applied to the treated surface and resin composite was light-cured, then examined using a universal testing machine. For acid resistance test, the specimens were immersed in acidic solution (pH 4.5), then examined under 3D confocal laser scanning microscope (CLSM). In µSBS, KF at 1,000, 9,000, and 10,000 ppm did not show differences compared with the control, while other concentrations of KF and NaF led to decreased µSBS. Higher concentrations of NaF and KF showed higher resistance to the acid challenge. So, we concluded that various concentrations of KF and NaF solutions had specific effects on µSBS and acid resistance. Keywords: Potassium fluoride, Sodium fluoride, Micro shear bonding strength, Acid resistance protective effect against erosive enamel loss compared INTRODUCTION with tooth mousses casein phosphopeptide-amorphous Dental caries is a degradation of tooth structure, which calciumphosphate (CPP-ACP)11). -

United States Patent Office E 8
United States Patent Office e 8. P t t 8 d s ep t. 6, 9 C 2 The addition compounds of phosphorus oxychloride 2,951,742 with niobium pentachloride and/or tantalum pentachlo PROCESS FOR THE RECOVERY OF METAL ride so obtained are solid compounds at ordinary tem HALDES FROM THER ADDUCTSWTH perature, which have lower melting points than those PHOSPHORUs oxYCHLORIDE of the pentachlorides used as starting materials. The constitution of the addition products has not been fully Waitersigao Scheier,to Ciba Neuewel, Limited, nearBasel, Basel, Switzerlaid, Switzerland, a Swiss as ascertained. However, analysis, has shown that there fina are formed, inter alia, 1:1-adducts of the metal penta chlorides with phosphorus oxychloride. The adducts are No Drawing. Filed Nov. 8, 1957, Ser. No. 695,229 O generally prepared industrially from mixtures of chlori Ciains priority, application Switzerland Nov. 13, 1956 nation products which are obtained, for example, by the chlorination of materials which contain niobium and 18 Claims. (C. 23-87) tantalum in oxidised form, for example, slags or espe cially concentrates or- ores, which may be after-treated This invention provides a process for the recovery 5 for the purpose of enrichment, or by the chlorination of of metal halides, especially chlorides of metals of the a mixtures of oxides of the aforesaid metals. The chlori fifth group of the periodic system, by splitting adducts nation of the aforesaid materials is carried out with of the metal halides with phosphorus oxychloride. chlorine gas and a reducing agent, such as carbon, or In patent application No. -

Potassium Fluoride As a Base in Organic Reactions
POTASSIUM FLUORIDE AS A BASE IN ORGANIC REACTIONS SOLUBILIZED BY 18-CROWN-6 A THESIS Presented to The Faculty of the Division of Graduate Studies by Thomas Ray Henson In Partial Fulfillment of the Requirements for the Degree Master of Science in Chemistry Georgia Institute of Technology March, 1975 POTASSIUM FLUORIDE AS A BASE IN ORGANIC REACTIONS SOLUBILIZED BY 18-CROWN-6 Approved: Charles Liotta, Chairman in^/ Grovenstein Date approved by Chairman £ UJgyuly 11*75 ACKNOWLEDGMENTS The author wishes to express his appreciation to his research director, Dr. C. L. Liotta, for the suggestion of this research problem and for his guidance and encouragement throughout the course of this work. The author also wishes to express his appreciation to Drs. E. Grovenstein and L. Zalkow for reading this thesis. The Department of Chemistry is gratefully acknowledged for financial support, as well as. Dr. C. L. Liotta. Finally, the author expresses a special word of thanks to his wife, Sandy, whose understanding and encouragement made this work possible. iii TABLE OF CONTENTS Page ACKNOWLEDGMENTS ii LIST OF TABLES iv LIST OF ILLUSTRATIONS v SUMMARY vi Chapter I. INTRODUCTION 1 Condensation Reactions Potassium Fluoride as a Base Crown Ethers II. EXPERIMENTAL 13 Chemicals 18-Crown-6 Synthesis Michael Condensations Alkylations 18-Crown-6 Complexes III. RESULTS AND DISCUSSIONS 47 Michael Condensations Knoevenagel Condensations Alkylations 18-Crown-6 Complexes IV. CONCLUSIONS 66 V. RECOMMENDATIONS 67 BIBLIOGRAPHY 68 iv LIST OF TABLES Table Page 1. Solubility of KF by 18-Crown-6 at 25° C 48 2. Michael Condensations Initiated by Potassium Fluoride in the Presence and Absence of 18-Crown-6 49 3. -

United States Patent Office Patented Nov
2,724,635 United States Patent Office Patented Nov. 22, 1955 1 2 completed, the solution is diluted and the insolubles such as silicates, etc., are removed by settling, decantation, 2,724,635 filtration, and the like. To the combined titanium iron PRODUCTION OF AN ALKALI METAL DOUBLE sulfate solution there is then admixed the alkali metal FLUORDE OF TTANIUM fluoride as from the afore-mentioned decomposition of Eugene Wainer, Cleveland Heights, Ohio, assignor, by double fluoride of titanium and alkali metal. Thus, where mesne assignments, to Horizons Titanium Corporatio), the decomposition has been in an electrolytic Zone, the Princeton, N.J., a corporation of New Jersey alkali fluoride remaining from the electrolysis is recycled to the zone reacting titanium compound with alkali metal No Drawing. Application February 4, 1952, 10 fluoride in the first instance. Usually, an electrolytic ; Serial No. 269,695 bath based on double fluoride of titanium, together with 5 Claims. (CI. 23-88) a molten bath constituent of halide, a chloride of alkali or alkaline earth metal (i. e., K, Na, Li, Sr, Ba, or mixtures, and preferably sodium chloride), after the In the production and use of compounds of titanium, electrolysis consists of potassium fluoride and sodium and particularly the fluoride compounds, a serious disad vantage heretofore has been the high cost of product by fluoride. Thus, with a bath in the first instance of potas reason of the expensive nature of the materials and pro ponentsium titanium subjected fluoride to electrolysis, and a sodium the decompositionchloride bath commay cedures which have been used. I have now found that be generally represented by the following equation: much of the high cost factor can be eliminated, and a 20. -

Potassium Fluoride Safety Data Sheet According to Federal Register / Vol
Potassium Fluoride Safety Data Sheet according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations Date of issue: 01/13/2014 Revision date: 01/31/2018 Supersedes: 01/31/2018 Version: 1.1 SECTION 1: Identification 1.1. Identification Product form : Substance Substance name : Potassium Fluoride CAS-No. : 7789-23-3 Product code : LC19090 Formula : KF Synonyms : Kaliumfluorid 1.2. Recommended use and restrictions on use Use of the substance/mixture : For laboratory and manufacturing use only. Recommended use : Laboratory chemicals Restrictions on use : Not for food, drug or household use 1.3. Supplier LabChem, Inc. Jackson's Pointe Commerce Park Building 1000, 1010 Jackson's Pointe Court Zelienople, PA 16063 - USA T 412-826-5230 - F 724-473-0647 1.4. Emergency telephone number Emergency number : CHEMTREC: 1-800-424-9300 or +1-703-741-5970 SECTION 2: Hazard(s) identification 2.1. Classification of the substance or mixture GHS-US classification Acute toxicity (oral) H301 Toxic if swallowed Category 3 Full text of H statements : see section 16 2.2. GHS Label elements, including precautionary statements GHS US labeling Hazard pictograms (GHS US) : GHS06 Signal word (GHS US) : Danger Hazard statements (GHS US) : H301 - Toxic if swallowed Precautionary statements (GHS US) : P264 - Wash exposed skin thoroughly after handling. P270 - Do not eat, drink or smoke when using this product. P301+P310 - IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P330 - If swallowed, rinse mouth P405 - Store locked up. P501 - Dispose of contents/container to comply with local, state and federal regulations 2.3. -

Introduction of Fluorine and Fluorine- Containing Functional Groups
Introduction of Fluorine and Fluorine- Containing Functional Groups The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Liang, Theresa, Constanze N. Neumann, and Tobias Ritter. 2013. Introduction of Fluorine and Fluorine-Containing Functional Groups. Angewandte Chemie International Edition 52, no. 32: 8214–8264. Published Version doi:10.1002/anie.201206566 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:12336403 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Open Access Policy Articles, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#OAP Fluorine DOI: 10.1002/anie.200((will be filled in by the editorial staff)) Introduction of Fluorine and Fluorine-Containing Functional Groups Theresa Liang, Constanze N. Neumann, and Tobias Ritter* Keywords: C–H functionalization fluorine catalysis trifluoromethylation transition metals 1 Theresa was born in 1985 in San Jose, A few decades ago, the development of several fluorinating California and received her undergraduate reagents such as Selectfluor®[11] and DAST (diethylaminosulfur education at University of California, [12] Berkeley pursuing research under the trifluoride) resulted in fast development of new fluorination mentorship of Prof. Richmond Sarpong in methods. Within the past ten years, a similar leap in fluorination total synthesis. After UC Berkeley, she chemistry has occurred, which we ascribe to increased efforts moved from sunny California to Harvard towards catalytic methods for fluorine incorporation. The merger of University and obtained her PhD in 2012 fluorination chemistry and synthetic organic chemistry––considered working with Prof. -

Introduction of Fluorine and Fluorine- Containing Functional Groups
Introduction of Fluorine and Fluorine- Containing Functional Groups The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Liang, Theresa, Constanze N. Neumann, and Tobias Ritter. 2013. Introduction of Fluorine and Fluorine-Containing Functional Groups. Angewandte Chemie International Edition 52, no. 32: 8214–8264. Published Version doi:10.1002/anie.201206566 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:12336403 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Open Access Policy Articles, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#OAP Fluorine DOI: 10.1002/anie.200((will be filled in by the editorial staff)) Introduction of Fluorine and Fluorine-Containing Functional Groups Theresa Liang, Constanze N. Neumann, and Tobias Ritter* Keywords: C–H functionalization fluorine catalysis trifluoromethylation transition metals 1 Theresa was born in 1985 in San Jose, A few decades ago, the development of several fluorinating California and received her undergraduate reagents such as Selectfluor®[11] and DAST (diethylaminosulfur education at University of California, [12] Berkeley pursuing research under the trifluoride) resulted in fast development of new fluorination mentorship of Prof. Richmond Sarpong in methods. Within the past ten years, a similar leap in fluorination total synthesis. After UC Berkeley, she chemistry has occurred, which we ascribe to increased efforts moved from sunny California to Harvard towards catalytic methods for fluorine incorporation. The merger of University and obtained her PhD in 2012 fluorination chemistry and synthetic organic chemistry––considered working with Prof. -

Resistance of Nickel-Containing Alloys in Hydrofluoric Acid, Hydrogen Fluoride and Fluorine (Ceb-5)
CORROSION- RESISTANCE OF NICKEL-CONTAINING ALLOYS IN HYDROFLUORIC ACID, HYDROGEN FLUORIDE AND FLUORINE (CEB-5) A PRACTICAL GUIDE TO THE USE OF NICKEL-CONTAINING ALLOYS NO 443 Distributed by Produced by NICKEL INCO INSTITUTE CORROSION-RESISTANCE OF NICKEL-CONTAINING ALLOYS IN HYDROFLUORIC ACID, HYDROGEN FLUORIDE AND FLUORINE (CEB-5) A PRACTICAL GUIDE TO THE USE OF NICKEL-CONTAINING ALLOYS NO 443 Originally, this handbook was published in 1976 by INCO, The International Nickel Company, Inc. Today this company is part of Vale S.A. The Nickel Institute republished the handbook in 2020. Despite the age of this publication the information herein is considered to be generally valid. Material presented in the handbook has been prepared for the general in-formation of the reader and should not be used or relied on for specific appli-cations without first securing competent advice. The Nickel Institute, the American Iron and Steel Institute, their members, staff and consultants do not represent or warrant its suitability for any general or specific use and assume no liability or responsibility of any kind in connec-tion with the information herein. Nickel Institute [email protected] www.nickelinstitute.org Table of Contents Page Introduction ............................................................................................................................................ 3 Corrosion by Hydrofluoric Acid .......................................................................................................... 3 Nickel-Copper