Facile Bioinspired Preparation of Fluorinase@Fluoridated Hydroxyapatite Nanoflowers 0 for the Biosynthesis of 5 -Fluorodeoxy Adenosine
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
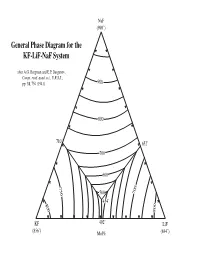
General Phase Diagram for the KF-Lif-Naf System
NaF (990˚) General Phase Diagram for the KF-LiF-NaF System after A.G. Bergman and E.P. Dergunov, Compt. rend. acad. sci., U.R.S.S., pp. 31, 754 (1941). 900 800 710˚ 652˚ 700 600 750 500 700 454˚ 800 800 KF 492˚ LiF (856˚) Mol% (844˚) Sodium Fluoride NaF 950˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 940˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 930˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 920˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 910˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 900˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 890˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 880˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 870˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 860˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 850˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 840˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 830˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 820˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 810˚ 810˚ ˚ KF 810 LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 800˚ 800˚ ˚ KF 800 LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 790˚ 790˚ ˚ KF 790 LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 780˚ 780˚ ˚ LiF KF 780 Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 770˚ 770˚ ˚ LiF KF 770 Potassium Fluoride -

Acidity, Basicity, and Pka 8 Connections
Acidity, basicity, and pKa 8 Connections Building on: Arriving at: Looking forward to: • Conjugation and molecular stability • Why some molecules are acidic and • Acid and base catalysis in carbonyl ch7 others basic reactions ch12 & ch14 • Curly arrows represent delocalization • Why some acids are strong and others • The role of catalysts in organic and mechanisms ch5 weak mechanisms ch13 • How orbitals overlap to form • Why some bases are strong and others • Making reactions selective using conjugated systems ch4 weak acids and bases ch24 • Estimating acidity and basicity using pH and pKa • Structure and equilibria in proton- transfer reactions • Which protons in more complex molecules are more acidic • Which lone pairs in more complex molecules are more basic • Quantitative acid/base ideas affecting reactions and solubility • Effects of quantitative acid/base ideas on medicine design Note from the authors to all readers This chapter contains physical data and mathematical material that some readers may find daunting. Organic chemistry students come from many different backgrounds since organic chemistry occu- pies a middle ground between the physical and the biological sciences. We hope that those from a more physical background will enjoy the material as it is. If you are one of those, you should work your way through the entire chapter. If you come from a more biological background, especially if you have done little maths at school, you may lose the essence of the chapter in a struggle to under- stand the equations. We have therefore picked out the more mathematical parts in boxes and you should abandon these parts if you find them too alien. -

The Heat of Combustion of Beryllium in Fluorine*
JOURNAL OF RESEARCH of the National Bureau of Standards -A. Physics and Chemistry Vol. 73A, No.3, May- June 1969 The Heat of Combustion of Beryllium in Fluorine* K. L. Churney and G. T. Armstrong Institute for Materials Research, National Bureau of Standards, Washington, D.C. 20234 (February 11, 1969) An expe rimental dete rmination of the e ne rgies of combustion in Auorine of polyte traAuoroethylene film and Q.o wder and of mixtures of beryllium with polytetraAuoroethyle ne gi ves for reacti on ( 1)f).H ~.or= - 1022.22 kJ 111 0 1- 1 (- 244.32 kcal mol - I) wit h a n ove ra ll precision of 0.96 kJ 111 0 1- 1 (0. 23 kcal 111 0 1- 1 ) at the 95 pe rce nt confid ence limit s. The tota l un cert a int y is estimated not to exceed ±3.2 kJ mol- I (±0.8 kcal mol - I). The measureme nts on polytetraflu oroeth yle ne giv e for reaction (2a) and reacti on (2 b) f).H ~. o c =- 10 369. 7 and - 10392.4 Jg- I, respective ly. Overall precisions e xpressed at the 95 pe rcent confide nce Ijmits are 3.3 and 6.0 Jg- I, respective ly. Be(c)+ F,(g) = BeF2(a morphous) (1) C,F.(polym e r powd er) + 2F2(g) = 2CF.(g) (2a) C2F.(polyme r film ) + 2F2 (g) = 2CF.(g) (2b) Be2C and Be metal were observed in a small carbonaceous residue from the co mbustion of the beryll iul11 -polytetraAuoroethylene mixtures. -

Trifluoromethane)
SAFETY DATA SHEET Halocarbon R-23 (Trifluoromethane) Section 1. Identification GHS product identifier : Halocarbon R-23 (Trifluoromethane) Chemical name : trifluoromethane Other means of : Fluoroform; Arcton 1; Fluoryl; Freon F-23; Freon 23; Genetron 23; Methyl trifluoride; R identification 23; Trifluoromethane; CHF3; Arcton; Halocarbon 23; UN 1984; Carbon trifluoride; Genetron HFC23; Propellant 23; Refrigerant 23 Product type : Liquefied gas Product use : Synthetic/Analytical chemistry. Synonym : Fluoroform; Arcton 1; Fluoryl; Freon F-23; Freon 23; Genetron 23; Methyl trifluoride; R 23; Trifluoromethane; CHF3; Arcton; Halocarbon 23; UN 1984; Carbon trifluoride; Genetron HFC23; Propellant 23; Refrigerant 23 SDS # : 001078 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Liquefied gas substance or mixture GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May cause frostbite. May displace oxygen and cause rapid suffocation. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. Use only equipment of compatible materials of construction. -

New and Improved Infrared Absorption Cross Sections for Chlorodifluoromethane (HCFC-22)
Atmos. Meas. Tech., 9, 2593–2601, 2016 www.atmos-meas-tech.net/9/2593/2016/ doi:10.5194/amt-9-2593-2016 © Author(s) 2016. CC Attribution 3.0 License. New and improved infrared absorption cross sections for chlorodifluoromethane (HCFC-22) Jeremy J. Harrison1,2 1Department of Physics and Astronomy, University of Leicester, University Road, Leicester, LE1 7RH, UK 2National Centre for Earth Observation, University of Leicester, University Road, Leicester, LE1 7RH, UK Correspondence to: Jeremy J. Harrison ([email protected]) Received: 10 December 2015 – Published in Atmos. Meas. Tech. Discuss.: 18 January 2016 Revised: 3 May 2016 – Accepted: 6 May 2016 – Published: 17 June 2016 Abstract. The most widely used hydrochlorofluorocarbon 1 Introduction (HCFC) commercially since the 1930s has been chloro- difluoromethane, or HCFC-22, which has the undesirable The consumer appetite for safe household refrigeration led effect of depleting stratospheric ozone. As this molecule to the commercialisation in the 1930s of dichlorodifluo- is currently being phased out under the Montreal Pro- romethane, or CFC-12, a non-flammable and non-toxic re- tocol, monitoring its concentration profiles using infrared frigerant (Myers, 2007). Within the next few decades, other sounders crucially requires accurate laboratory spectroscopic chemically related refrigerants were additionally commer- data. This work describes new high-resolution infrared ab- cialised, including chlorodifluoromethane, or a hydrochlo- sorption cross sections of chlorodifluoromethane over the rofluorocarbon known as HCFC-22, which found use in a spectral range 730–1380 cm−1, determined from spectra wide array of applications such as air conditioners, chillers, recorded using a high-resolution Fourier transform spectrom- and refrigeration for food retail and industrial processes. -

Fluoroform (CHF3)
SYNLETT0936-52141437-2096 © Georg Thieme Verlag Stuttgart · New York 2015, 26, 1911–1912 1911 spotlight Syn lett S. Kyasa Spotlight Fluoroform (CHF3) ShivaKumar Kyasa ShivaKumar Kyasa was born in Telangana state, India, in 1978. After completing a B.Sc. (chemis- try, biology) and a M.Sc. (medicinal chemistry) Department of Chemistry, University of Nebraska-Lincoln, Lincoln, NE 68588-0304, USA from Osmania University, he worked at Dr. Red- [email protected] dy’s Laboratories, Ltd., Hyderabad, India. He is currently pursuing a Ph.D. in chemistry at the Published online: 11.06.2015 University of Nebraska–Lincoln, USA under the DOI: 10.1055/s-0034-1380924; Art ID: st-2015-v0519-v supervision of Prof. Patrick H. Dussault. His re- search focuses on C–O bond formation and syn- thesis of functionalized ethers using peroxide oxygen as an electrophile. Introduction with Lewis bases (most commonly catalytic fluoride) to af- ford pentavalent silicon species as nucleophiles has enabled Fluoroform, which is generated in ~20 kilotons/year trifluoromethylation of a number of electrophiles.4 The tri- as a side product of Teflon manufacture,1,2 is a low-boiling fluoromethyl group has great importance in medicinal, ag- (-82 °C), non-toxic and non-ozone-depleting gas.1,3 Howev- rochemical and materials science.5 This spotlight describes er, fluoroform is a potent greenhouse agent and there is recently reported methods for tri- and difluoromethylation great interest in methods for use of the gas as a synthetic based upon fluoroform. reagent. The direct use of trifluoromethyl anion as a nucleo- phile has been challenging due to facile α-elimination to CF3 CF2 + F fluoride and difluorocarbene (Scheme 1). -

SAFETY DATA SHEET Halocarbon R-503
SAFETY DATA SHEET Halocarbon R-503 Section 1. Identification GHS product identifier : Halocarbon R-503 Other means of : Not available. identification Product type : Liquefied gas Product use : Synthetic/Analytical chemistry. SDS # : 007306 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Liquefied gas substance or mixture HAZARDOUS TO THE OZONE LAYER - Category 1 GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May cause frostbite. May displace oxygen and cause rapid suffocation. Harms public health and the environment by destroying ozone in the upper atmosphere. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. Use only equipment of compatible materials of construction. Always keep container in upright position. Prevention : Not applicable. Response : Not applicable. Storage : Protect from sunlight. Store in a well-ventilated place. Disposal : Refer to manufacturer or supplier for information on recovery or recycling. Hazards not otherwise : Liquid can cause burns similar to frostbite. -

United States Patent Office Patented July 1, 1969
3,453,337 United States Patent Office Patented July 1, 1969 1. 2 3,453,337 FLUORINATION OF HALOGENATED The presence in the reaction mixture of the two fluo ORGANIC COMPOUNDS rides, or the complex fluoride enables better yields of Royston Henry Bennett and David Walter Cottrell, Ayon highly fluorinated products to be obtained under less mouth, England, assignors to Imperial Smelting Cor severe reaction condions, markedly increases the amount poration (N.S.C.) Limited, London, England, a British of fluorination reagent reacted under otherwise similar company conditions and enables the fluorination reaction to be No brawing. Filed Feb. 19, 1965, Ser. No. 434,128 carried out (for the same yield of product) at a lower Claims priority, application Great Britain, Feb. 26, 1964, temperature with the consequent use of less costly ma 7,932/64 terials and techniques of reactor construction. The pres Int, C. C07c 25/04 10 ence of the fluorides enables the vapor phase reaction U.S. C. 260-650 2 Claims to be carried out (for the same yields) at lower pressure This invention relates to the fluorination of organic than the pressure involved in the reactions using only halogen compounds and more especially to a process the alkali metal fluorides as proposed hitherto. The fur for the production of highly fluorinated aromatic com ther possibility of using a continuous flow apparatus such pounds by the replacement of higher halogen atoms in 15 as a fluidised reactor will be apparent to those familiar halogeno-aromatic compounds by fluorine atoms. with the art. The presence of the two fluorides or com Aromatic halogenocarbons containing carbon and halo plex fluoride enables a lower temperature to be em gen atoms only can be reacted with alkali fluorides ployed than was hitherto believed to be necessary, with under various conditions to give yields of halofluoro a consequent reduction in the extent of thermal degrada aromatic compounds. -

Calcium Fluoride Scale Prevention
Tech Manual Excerpt Water Chemistry and Pretreatment Calcium Fluoride Scale Prevention Calcium Fluoride Fluoride levels in the feedwater of as low as 0.1 mg/L can create a scaling potential if Scale Prevention the calcium concentration is high. The calculation of the scaling potential is analogous to the procedure described in Calcium Sulfate Scale Prevention (Form No. 45-D01553-en) for CaSO4. Calculation 1. Calculate the ionic strength of the concentrate stream (Ic) following the procedure described in Scaling Calculations (Form No. 45-D01551-en): Eq. 1 2. Calculate the ion product (IPc) for CaF2 in the concentrate stream: Eq. 2 where: m 2+ 2+ ( Ca )f = M Ca in feed, mol/L m – – ( F )f = M F in feed, mol/L 3. Compare IPc for CaF2 with the solubility product (Ksp) of CaF2 at the ionic strength of the concentrate stream, Figure 2 /11/. If IPc ≥ Ksp, CaF2 scaling can occur, and adjustment is required. Adjustments for CaF2 Scale Control The adjustments discussed in Calcium Sulfate Scale Prevention (Form No. 45-D01553-en) for CaSO4 scale control apply as well for CaF2 scale control. Page 1 of 3 Form No. 45-D01556-en, Rev. 4 April 2020 Figure 1: Ksp for SrSO4 versus ionic strength /10/ Page 2 of 3 Form No. 45-D01556-en, Rev. 4 April 2020 Figure 2: Ksp for CaF2 versus ionic strength /11/ Excerpt from FilmTec™ Reverse Osmosis Membranes Technical Manual (Form No. 45-D01504-en), Chapter 2, "Water Chemistry and Pretreatment." Have a question? Contact us at: All information set forth herein is for informational purposes only. -

Environmental Impact Assessment and Due Diligence Report (DRAFT)
Environmental Impact Assessment and Due Diligence Report (DRAFT) Project Number: 47051-002 May 2015 PRC: Chemical Industry Energy Efficiency and Emissions Reduction Project Prepared by ChemChina and CHC for the Asian Development Bank. This is a version of the draft posted in May 2015 available on http://www.adb.org/Documents/RRPs/?id=47051-002-3. CURRENCY EQUIVALENTS (as of 11 May 2015) Currency unit – yuan (CNY) CNY1.00 = $ 0.1633 $1.00 = CNY 6.1254 ABBREVIATIONS ADB – Asian Development Bank AP – Affected Person API – American Petroleum Institute ASL – Above Sea Level CCPS – Center for Chemical Process Safety CEMS – Continuous Emissions Monitoring System ChemChina – China National Chemical Corporation CHC – China Haohua Company CHP – Combined Heat and Power CNY – Chinese Yuan CSEMP – Construction Site Environmental Management Plan DCS – Distributed Control System EA – Executing Agency EHS – Environment, Health and Safety EHSS – Environment, Health and Safety Specialist EHSU – Environment, Health and Safety Unit EIA – Environmental Impact Assessment EMoP – Environmental Monitoring Plan EMP – Environmental Management Plan EMS – Environmental Monitoring Station EPB – Environmental Protection Bureau FSR – Feasibility Study Report Flor – Calcium fluoride (CaF2) or “fluorite” GDP – Gross Domestic Product GHG – Greenhouse Gas GIP – Good International Practice GRM – Grievance Redress Mechanism HDP – High Density Polyethylene IA – Implementing Agency IEE – Initial Environmental Examination IT – Interim Target LIC – Loan Implementation Consultant -

SAFETY DATA SHEET Halocarbon R-23 (Trifluoromethane)
SAFETY DATA SHEET Halocarbon R-23 (Trifluoromethane) Section 1. Identification GHS product identifier : Halocarbon R-23 (Trifluoromethane) Chemical name : trifluoromethane Other means of : Fluoroform; Arcton 1; Fluoryl; Freon F-23; Freon 23; Genetron 23; Methyl trifluoride; R identification 23; Trifluoromethane; CHF3; Arcton; Halocarbon 23; UN 1984; Carbon trifluoride; Genetron HFC23; Propellant 23; Refrigerant 23 Product use : Synthetic/Analytical chemistry. Synonym : Fluoroform; Arcton 1; Fluoryl; Freon F-23; Freon 23; Genetron 23; Methyl trifluoride; R 23; Trifluoromethane; CHF3; Arcton; Halocarbon 23; UN 1984; Carbon trifluoride; Genetron HFC23; Propellant 23; Refrigerant 23 SDS # : 000023 Supplier's details : ASPEN Refrigerants, Inc. 38-18 33rd Street Long Island City, NY 11101 1-800-473-3766 Emergency telephone :1-800-424-9300 number (with 24 hours of operation) Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Liquefied gas substance or mixture GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May cause frostbite. May displace oxygen and cause rapid suffocation. Precautionary statements General : Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Prevention : Use and store only outdoors or in a well-ventilated place. Response : Not applicable. Storage : Protect from sunlight. Store in a well-ventilated place. Disposal : Not applicable. Hazards not otherwise : Liquid can cause burns similar to frostbite. May displace oxygen and cause rapid classified suffocation. Date of issue/Date of revision 2/1/2019 Date of previous issue : No previous validation. -

Utilization of Fluoroform for Difluoromethylation in Continuous
Green Chemistry View Article Online COMMUNICATION View Journal | View Issue Utilization of fluoroform for difluoromethylation in continuous flow: a concise synthesis of Cite this: Green Chem., 2018, 20, 108 α-difluoromethyl-amino acids† Received 26th September 2017, Accepted 30th October 2017 Manuel Köckinger,a Tanja Ciaglia,a Michael Bersier,b Paul Hanselmann,b DOI: 10.1039/c7gc02913f Bernhard Gutmann *a,c and C. Oliver Kappe *a,c rsc.li/greenchem Fluoroform (CHF3) can be considered as an ideal reagent for trolled under the Montreal Protocol. As a consequence, its pro- difluoromethylation reactions. However, due to the low reactivity duction and usage has become increasingly limited and expen- of fluoroform, only very few applications have been reported so sive. A plethora of alternative difluoromethane sources have far. Herein we report a continuous flow difluoromethylation proto- been developed in recent years, including TMSCF2Br, 3 2 col on sp carbons employing fluoroform as a reagent. The proto- (EtO)2POCF2Br, PhCOCF2Cl and CHF2OTf. Although these col is applicable for the direct Cα-difluoromethylation of protected reagents cover the needs of chemists for difluoromethylation Creative Commons Attribution 3.0 Unported Licence. α-amino acids, and enables a highly atom efficient synthesis of the on a laboratory scale, their high cost, low atom economy and active pharmaceutical ingredient eflornithine. limited commercial availability prohibit their usage in an industrial setting. The difluoromethyl group is found in an increasing array of The most attractive CF - and CHF -source is fluoroform 1 3 2 pharmaceutical and agrochemical products. Not surprisingly, (CHF , Freon 23). Fluoroform is generated as a large-volume ff 3 therefore, significant e orts have been devoted towards the waste-product during the synthesis of chlorodifluoromethane development of novel protocols for the introduction of the (Fig.