New and Improved Infrared Absorption Cross Sections for Chlorodifluoromethane (HCFC-22)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acidity, Basicity, and Pka 8 Connections
Acidity, basicity, and pKa 8 Connections Building on: Arriving at: Looking forward to: • Conjugation and molecular stability • Why some molecules are acidic and • Acid and base catalysis in carbonyl ch7 others basic reactions ch12 & ch14 • Curly arrows represent delocalization • Why some acids are strong and others • The role of catalysts in organic and mechanisms ch5 weak mechanisms ch13 • How orbitals overlap to form • Why some bases are strong and others • Making reactions selective using conjugated systems ch4 weak acids and bases ch24 • Estimating acidity and basicity using pH and pKa • Structure and equilibria in proton- transfer reactions • Which protons in more complex molecules are more acidic • Which lone pairs in more complex molecules are more basic • Quantitative acid/base ideas affecting reactions and solubility • Effects of quantitative acid/base ideas on medicine design Note from the authors to all readers This chapter contains physical data and mathematical material that some readers may find daunting. Organic chemistry students come from many different backgrounds since organic chemistry occu- pies a middle ground between the physical and the biological sciences. We hope that those from a more physical background will enjoy the material as it is. If you are one of those, you should work your way through the entire chapter. If you come from a more biological background, especially if you have done little maths at school, you may lose the essence of the chapter in a struggle to under- stand the equations. We have therefore picked out the more mathematical parts in boxes and you should abandon these parts if you find them too alien. -

Effect of Insulations on Cop in Vapor Compression Refrigeration System
International Journal of Mechanical Engineering and Technology (IJMET) Volume 10, Issue 01, January 2019, pp. 1201-1208, Article ID: IJMET_10_01_122 Available online at http://www.iaeme.com/ijmet/issues.asp?JType=IJMET&VType=10&IType= 01 ISSN Print: 0976-6340 and ISSN Online: 0976-6359 © IAEME Publication Scopus Indexed EFFECT OF INSULATIONS ON COP IN VAPOR COMPRESSION REFRIGERATION SYSTEM Anusha Peyyala Assistant Professor P V P Siddhartha Institute of Technology, Research Scholar, Acharya Nagarjuna University, India. Dr N V V S Sudheer Associate Professor, R V R & J C College of Engineering, Guntur India ABSTRACT In this project, experimentation is done on Vapour Compression Refrigeration System [VCRS] as the COP is high for this system and it is the present trend of the HVAC in the domestic industry. This study presents investigation of best suited refrigerant and insulation combination for gas pipeline and liquid pipeline of a split air conditioning system. Analysis are performed for R22-Chlorodiflouromethane, a HydroChloroFlouro Carbon refrigerant, which has been using in the present world that cause both global warming and ozone layer depletion and R410a, mixture of di- flouromethane and pentaflouroethane, a Hydroflouro carbon refrigerant, which is future of HVAC which reduces the effect of ozone layer depletion [ODP] and Global Warming Potential [GWP].For these two refrigerants, we had found out the best insulation suitable as insulation also affects the COP of air conditioner, which has been observed from the literature. Minimizing the temperature of refrigerant in suction line helps condensing unit work more effectively intern the system performance increases. This reduces the overall power required for working of air conditioner, thereby reducing the maintenance cost of system. -

Trifluoromethane)
SAFETY DATA SHEET Halocarbon R-23 (Trifluoromethane) Section 1. Identification GHS product identifier : Halocarbon R-23 (Trifluoromethane) Chemical name : trifluoromethane Other means of : Fluoroform; Arcton 1; Fluoryl; Freon F-23; Freon 23; Genetron 23; Methyl trifluoride; R identification 23; Trifluoromethane; CHF3; Arcton; Halocarbon 23; UN 1984; Carbon trifluoride; Genetron HFC23; Propellant 23; Refrigerant 23 Product type : Liquefied gas Product use : Synthetic/Analytical chemistry. Synonym : Fluoroform; Arcton 1; Fluoryl; Freon F-23; Freon 23; Genetron 23; Methyl trifluoride; R 23; Trifluoromethane; CHF3; Arcton; Halocarbon 23; UN 1984; Carbon trifluoride; Genetron HFC23; Propellant 23; Refrigerant 23 SDS # : 001078 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Liquefied gas substance or mixture GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May cause frostbite. May displace oxygen and cause rapid suffocation. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. Use only equipment of compatible materials of construction. -

Fluoroform (CHF3)
SYNLETT0936-52141437-2096 © Georg Thieme Verlag Stuttgart · New York 2015, 26, 1911–1912 1911 spotlight Syn lett S. Kyasa Spotlight Fluoroform (CHF3) ShivaKumar Kyasa ShivaKumar Kyasa was born in Telangana state, India, in 1978. After completing a B.Sc. (chemis- try, biology) and a M.Sc. (medicinal chemistry) Department of Chemistry, University of Nebraska-Lincoln, Lincoln, NE 68588-0304, USA from Osmania University, he worked at Dr. Red- [email protected] dy’s Laboratories, Ltd., Hyderabad, India. He is currently pursuing a Ph.D. in chemistry at the Published online: 11.06.2015 University of Nebraska–Lincoln, USA under the DOI: 10.1055/s-0034-1380924; Art ID: st-2015-v0519-v supervision of Prof. Patrick H. Dussault. His re- search focuses on C–O bond formation and syn- thesis of functionalized ethers using peroxide oxygen as an electrophile. Introduction with Lewis bases (most commonly catalytic fluoride) to af- ford pentavalent silicon species as nucleophiles has enabled Fluoroform, which is generated in ~20 kilotons/year trifluoromethylation of a number of electrophiles.4 The tri- as a side product of Teflon manufacture,1,2 is a low-boiling fluoromethyl group has great importance in medicinal, ag- (-82 °C), non-toxic and non-ozone-depleting gas.1,3 Howev- rochemical and materials science.5 This spotlight describes er, fluoroform is a potent greenhouse agent and there is recently reported methods for tri- and difluoromethylation great interest in methods for use of the gas as a synthetic based upon fluoroform. reagent. The direct use of trifluoromethyl anion as a nucleo- phile has been challenging due to facile α-elimination to CF3 CF2 + F fluoride and difluorocarbene (Scheme 1). -

SAFETY DATA SHEET Chlorodifluoromethane (R 22) Issue Date: 16.01.2013 Version: 1.0 SDS No.: 000010021746 Last Revised Date: 18.06.2015 1/15
SAFETY DATA SHEET Chlorodifluoromethane (R 22) Issue Date: 16.01.2013 Version: 1.0 SDS No.: 000010021746 Last revised date: 18.06.2015 1/15 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier Product name: Chlorodifluoromethane (R 22) Trade name: Gasart 503 R22 Additional identification Chemical name: Chlorodifluoromethane Chemical formula: CHClF2 INDEX No. - CAS-No. 75-45-6 EC No. 200-871-9 REACH Registration No. 01-2119517587-31 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses: Industrial and professional. Perform risk assessment prior to use. Refrigerant. Using gas alone or in mixtures for the calibration of analysis equipment. Using gas as feedstock in chemical processes. Formulation of mixtures with gas in pressure receptacles. Uses advised against Consumer use. 1.3 Details of the supplier of the safety data sheet Supplier Linde Gas GmbH Telephone: +43 50 4273 Carl-von-Linde-Platz 1 A-4651 Stadl-Paura E-mail: [email protected] 1.4 Emergency telephone number: Emergency number Linde: + 43 50 4273 (during business hours), Poisoning Information Center: +43 1 406 43 43 SDS_AT - 000010021746 SAFETY DATA SHEET Chlorodifluoromethane (R 22) Issue Date: 16.01.2013 Version: 1.0 SDS No.: 000010021746 Last revised date: 18.06.2015 2/15 SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Directive 67/548/EEC or 1999/45/EC as amended. N; R59 The full text for all R-phrases is displayed in section 16. Classification according to Regulation (EC) No 1272/2008 as amended. -

SAFETY DATA SHEET Halocarbon R-503
SAFETY DATA SHEET Halocarbon R-503 Section 1. Identification GHS product identifier : Halocarbon R-503 Other means of : Not available. identification Product type : Liquefied gas Product use : Synthetic/Analytical chemistry. SDS # : 007306 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Liquefied gas substance or mixture HAZARDOUS TO THE OZONE LAYER - Category 1 GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May cause frostbite. May displace oxygen and cause rapid suffocation. Harms public health and the environment by destroying ozone in the upper atmosphere. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. Use only equipment of compatible materials of construction. Always keep container in upright position. Prevention : Not applicable. Response : Not applicable. Storage : Protect from sunlight. Store in a well-ventilated place. Disposal : Refer to manufacturer or supplier for information on recovery or recycling. Hazards not otherwise : Liquid can cause burns similar to frostbite. -

UNITED STATES PATENT of FICE 2,640,086 PROCESS for SEPARATING HYDROGEN FLUORIDE from CHLORODFLUORO METHANE Robert H
Patented May 26, 1953 2,640,086 UNITED STATES PATENT of FICE 2,640,086 PROCESS FOR SEPARATING HYDROGEN FLUORIDE FROM CHLORODFLUORO METHANE Robert H. Baldwin, Chadds Ford, Pa., assignor to E. H. du Pont de Nemours and Company, Wi inington, Del, a corporation of Delaware No Drawing. Application December 15, 1951, Serial No. 261,929 9 Claims. (C. 260-653) 2 This invention relates to a process for Sep These objects are accomplished essentially by arating hydrogen fluoride from monochlorodi Subjecting a mixture of hydrogen fluoride and fluoronethane, and more particularly, separat Inonochlorodifluoromethane in the liquid phase ing these components from the reaction mixture to temperatures below 0° C., preferably at about obtained in the fluorination of chloroform with -30° C. to -50° C., at either atmospheric or hydrogen fluoride, Super-atmospheric pressures, together with from In the fluorination of chloroform in the prest about 0.25 mol to about 2.5 mols of chloroform ence Of a Catalyst, a reaction mixture is pro per mol of chlorodifluoronethane contained in duced which consists essentially of HCl, HF, the mixture and separating an upper layer rich CHCIF2, CHCl2F, CHCls, and CHF3. A method O in HF from a lower organic layer. The proceSS of Separating these components is disclosed in is operative with mixtures containing up to 77% U. S. Patent No. 2,450,414 which involves sep by weight of HF. arating the components by a special fractional It has been found that chloroform is substan distillation under appropriate temperatures and tially immiscible With EIF at temperatures be pressures. -

CYCLE D-HX: NIST Vapor Compression Cycle Model Accounting for Refrigerant Thermodynamic and Transport Properties
NIST Technical Note 1974 CYCLE_D-HX: NIST Vapor Compression Cycle Model Accounting for Refrigerant Thermodynamic and Transport Properties Version 1.0 User’s Guide J.S. Brown R. Brignoli P.A. Domanski This publication is available free of charge from: https://doi.org/10.6028/NIST.TN.1974 NIST Technical Note 1974 CYCLE_D-HX: NIST Vapor Compression Cycle Model Accounting for Refrigerant Thermodynamic and Transport Properties Version 1.0 User’s Guide J.S. Brown The Catholic University of America R. Brignoli P.A. Domanski Engineering Laboratory National Institute of Standards and Technology This publication is available free of charge from: https://doi.org/10.6028/NIST.TN.1974 December 2017 U.S. Department of Commerce Wilbur L. Ross, Jr., Secretary National Institute of Standards and Technology Walter Copan, NIST Director and Under Secretary of Commerce for Standards and Technology This software package was developed by the National Institute of Standards and Technology (NIST), is not subject to copyright protection, and is in the public domain. It can be used freely provided that any derivative works bear some notice that they are derived from it. NIST used its best efforts to provide a high-quality software package and to select modeling methods and correlations based on sound scientific judgement. However, NIST assumes neither responsibility nor liability for any damage arising out of or relating to the use of CYCLE_D-HX. The software is provided “AS IS”; NIST makes NO GUARANTIES and NO WARRANTIES OF ANY TYPE, expressed or implied, including NO WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. -
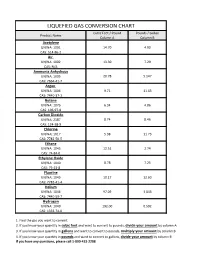
Liquefied Gas Conversion Chart
LIQUEFIED GAS CONVERSION CHART Cubic Feet / Pound Pounds / Gallon Product Name Column A Column B Acetylene UN/NA: 1001 14.70 4.90 CAS: 514-86-2 Air UN/NA: 1002 13.30 7.29 CAS: N/A Ammonia Anhydrous UN/NA: 1005 20.78 5.147 CAS: 7664-41-7 Argon UN/NA: 1006 9.71 11.63 CAS: 7440-37-1 Butane UN/NA: 1075 6.34 4.86 CAS: 106-97-8 Carbon Dioxide UN/NA: 2187 8.74 8.46 CAS: 124-38-9 Chlorine UN/NA: 1017 5.38 11.73 CAS: 7782-50-5 Ethane UN/NA: 1045 12.51 2.74 CAS: 74-84-0 Ethylene Oxide UN/NA: 1040 8.78 7.25 CAS: 75-21-8 Fluorine UN/NA: 1045 10.17 12.60 CAS: 7782-41-4 Helium UN/NA: 1046 97.09 1.043 CAS: 7440-59-7 Hydrogen UN/NA: 1049 192.00 0.592 CAS: 1333-74-0 1. Find the gas you want to convert. 2. If you know your quantity in cubic feet and want to convert to pounds, divide your amount by column A 3. If you know your quantity in gallons and want to convert to pounds, multiply your amount by column B 4. If you know your quantity in pounds and want to convert to gallons, divide your amount by column B If you have any questions, please call 1-800-433-2288 LIQUEFIED GAS CONVERSION CHART Cubic Feet / Pound Pounds / Gallon Product Name Column A Column B Hydrogen Chloride UN/NA: 1050 10.60 8.35 CAS: 7647-01-0 Krypton UN/NA: 1056 4.60 20.15 CAS: 7439-90-9 Methane UN/NA: 1971 23.61 3.55 CAS: 74-82-8 Methyl Bromide UN/NA: 1062 4.03 5.37 CAS: 74-83-9 Neon UN/NA: 1065 19.18 10.07 CAS: 7440-01-9 Mapp Gas UN/NA: 1060 9.20 4.80 CAS: N/A Nitrogen UN/NA: 1066 13.89 6.75 CAS: 7727-37-9 Nitrous Oxide UN/NA: 1070 8.73 6.45 CAS: 10024-97-2 Oxygen UN/NA: 1072 12.05 9.52 CAS: 7782-44-7 Propane UN/NA: 1075 8.45 4.22 CAS: 74-98-6 Sulfur Dioxide UN/NA: 1079 5.94 12.0 CAS: 7446-09-5 Xenon UN/NA: 2036 2.93 25.51 CAS: 7440-63-3 1. -

R-22 Safety Data Sheet
R-22 Safety Data Sheet R-22 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME: R-22 OTHER NAME: Chlorodifluoromethane USE: Refrigerant Gas DISTRIBUTOR: National Refrigerants, Inc. 661 Kenyon Avenue Bridgeton, New Jersey 08302 FOR MORE INFORMATION CALL: IN CASE OF EMERGENCY CALL: (Monday-Friday, 8:00am-5:00pm) CHEMTREC: 1-800-424-9300 1-800-262-0012 2. HAZARDS IDENTIFICATION CLASSIFICATION: Gases under pressure, Liquefied Gas SIGNAL WORD: WARNING HAZARD STATEMENT: Contains gas under pressure, may explode if heated SYMBOL: Gas Cylinder PRECAUTIONARY STATEMENT: STORAGE: Protect from sunlight, store in a well ventilated place EMERGENCY OVERVIEW: Colorless, volatile liquid with ethereal and faint sweetish odor. Non-flammable material. Overexposure may cause dizziness and loss of concentration. At higher levels, CNS depression and cardiac arrhythmia may result from exposure. Vapors displace air and can cause asphyxiation in confined spaces. At higher temperatures, (>250C), decomposition products may include Hydrochloric Acid (HCI), Hydrofluoric Acid (HF) and carbonyl halides. POTENTIAL HEALTH HAZARDS SKIN: Irritation would result from a defatting action on tissue. Liquid contact could cause frostbite. EYES: Liquid contact can cause severe irritation and frostbite. Mist may irritate. INHALATION: R-22 is low in acute toxicity in animals. When oxygen levels in air are reduced to 12-14% by displacement, symptoms of asphyxiation, loss of coordination, increased pulse rate and deeper respiration will occur. At high levels, cardiac arrhythmia may occur. INGESTION: Ingestion is unlikely because of the low boiling point of the material. Should it occur, discomfort in the gastrointestinal tract from rapid evaporation of the material and consequent evolution of gas would result. -

181 Chloroform 4. Production, Import/Export, Use, And
CHLOROFORM 181 4. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL Chloroform is used primarily in the production of chlorodifluoromethane (hydrochlorofluorocarbon-22 or HCFC-22) used as a refrigerant for home air conditioners or large supermarket freezers and in the production of fluoropolymers (CMR 1995). Chloroform has also been used as a solvent, a heat transfer medium in fire extinguishers, an intermediate in the preparation of dyes and pesticides, and other applications highlighted below. Its use as an anesthetic has been largely discontinued. It has limited medical uses in some dental procedures and in the administration of drugs for the treatment of some diseases. 4.1 PRODUCTION The chlorination of methane and the chlorination of methyl chloride produced by the reaction of methanol and hydrogen chloride are the two common methods for commercial chloroform production (Ahlstrom and Steele 1979; Deshon 1979). The Vulcan Materials Co., Wichita, Kansas, was documented as still using the methanol production process during the late 1980s with all other facilities in the United States at that time using the methyl chloride chlorination process (SRI 1990). One U.S. manufacturer began chloroform production in 1903, but significant commercial production was not reported until 1922 (IARC 1979). Since the early 1980s, chloroform production increased by 20-25%, due primarily to a higher demand for HCFC-22, the major chemical produced from chloroform. The Montreal Protocol established goals for phasing out the use of a variety of ozonedepleting chemicals, including most chlorofluorocarbons (CFCs). HCFC-22 was one of the few fluorocarbons not restricted by the international agreement. Chloroform is used in the manufacture of HCFC-22, and an increase in the production of this refrigerant has led to a modest increase in the demand for chloroform (CMR 1989). -

SAFETY DATA SHEET Halocarbon R-23 (Trifluoromethane)
SAFETY DATA SHEET Halocarbon R-23 (Trifluoromethane) Section 1. Identification GHS product identifier : Halocarbon R-23 (Trifluoromethane) Chemical name : trifluoromethane Other means of : Fluoroform; Arcton 1; Fluoryl; Freon F-23; Freon 23; Genetron 23; Methyl trifluoride; R identification 23; Trifluoromethane; CHF3; Arcton; Halocarbon 23; UN 1984; Carbon trifluoride; Genetron HFC23; Propellant 23; Refrigerant 23 Product use : Synthetic/Analytical chemistry. Synonym : Fluoroform; Arcton 1; Fluoryl; Freon F-23; Freon 23; Genetron 23; Methyl trifluoride; R 23; Trifluoromethane; CHF3; Arcton; Halocarbon 23; UN 1984; Carbon trifluoride; Genetron HFC23; Propellant 23; Refrigerant 23 SDS # : 000023 Supplier's details : ASPEN Refrigerants, Inc. 38-18 33rd Street Long Island City, NY 11101 1-800-473-3766 Emergency telephone :1-800-424-9300 number (with 24 hours of operation) Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Liquefied gas substance or mixture GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May cause frostbite. May displace oxygen and cause rapid suffocation. Precautionary statements General : Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Prevention : Use and store only outdoors or in a well-ventilated place. Response : Not applicable. Storage : Protect from sunlight. Store in a well-ventilated place. Disposal : Not applicable. Hazards not otherwise : Liquid can cause burns similar to frostbite. May displace oxygen and cause rapid classified suffocation. Date of issue/Date of revision 2/1/2019 Date of previous issue : No previous validation.