Fluoroform (CHF3)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acidity, Basicity, and Pka 8 Connections
Acidity, basicity, and pKa 8 Connections Building on: Arriving at: Looking forward to: • Conjugation and molecular stability • Why some molecules are acidic and • Acid and base catalysis in carbonyl ch7 others basic reactions ch12 & ch14 • Curly arrows represent delocalization • Why some acids are strong and others • The role of catalysts in organic and mechanisms ch5 weak mechanisms ch13 • How orbitals overlap to form • Why some bases are strong and others • Making reactions selective using conjugated systems ch4 weak acids and bases ch24 • Estimating acidity and basicity using pH and pKa • Structure and equilibria in proton- transfer reactions • Which protons in more complex molecules are more acidic • Which lone pairs in more complex molecules are more basic • Quantitative acid/base ideas affecting reactions and solubility • Effects of quantitative acid/base ideas on medicine design Note from the authors to all readers This chapter contains physical data and mathematical material that some readers may find daunting. Organic chemistry students come from many different backgrounds since organic chemistry occu- pies a middle ground between the physical and the biological sciences. We hope that those from a more physical background will enjoy the material as it is. If you are one of those, you should work your way through the entire chapter. If you come from a more biological background, especially if you have done little maths at school, you may lose the essence of the chapter in a struggle to under- stand the equations. We have therefore picked out the more mathematical parts in boxes and you should abandon these parts if you find them too alien. -

Trifluoromethane)
SAFETY DATA SHEET Halocarbon R-23 (Trifluoromethane) Section 1. Identification GHS product identifier : Halocarbon R-23 (Trifluoromethane) Chemical name : trifluoromethane Other means of : Fluoroform; Arcton 1; Fluoryl; Freon F-23; Freon 23; Genetron 23; Methyl trifluoride; R identification 23; Trifluoromethane; CHF3; Arcton; Halocarbon 23; UN 1984; Carbon trifluoride; Genetron HFC23; Propellant 23; Refrigerant 23 Product type : Liquefied gas Product use : Synthetic/Analytical chemistry. Synonym : Fluoroform; Arcton 1; Fluoryl; Freon F-23; Freon 23; Genetron 23; Methyl trifluoride; R 23; Trifluoromethane; CHF3; Arcton; Halocarbon 23; UN 1984; Carbon trifluoride; Genetron HFC23; Propellant 23; Refrigerant 23 SDS # : 001078 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Liquefied gas substance or mixture GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May cause frostbite. May displace oxygen and cause rapid suffocation. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. Use only equipment of compatible materials of construction. -

New and Improved Infrared Absorption Cross Sections for Chlorodifluoromethane (HCFC-22)
Atmos. Meas. Tech., 9, 2593–2601, 2016 www.atmos-meas-tech.net/9/2593/2016/ doi:10.5194/amt-9-2593-2016 © Author(s) 2016. CC Attribution 3.0 License. New and improved infrared absorption cross sections for chlorodifluoromethane (HCFC-22) Jeremy J. Harrison1,2 1Department of Physics and Astronomy, University of Leicester, University Road, Leicester, LE1 7RH, UK 2National Centre for Earth Observation, University of Leicester, University Road, Leicester, LE1 7RH, UK Correspondence to: Jeremy J. Harrison ([email protected]) Received: 10 December 2015 – Published in Atmos. Meas. Tech. Discuss.: 18 January 2016 Revised: 3 May 2016 – Accepted: 6 May 2016 – Published: 17 June 2016 Abstract. The most widely used hydrochlorofluorocarbon 1 Introduction (HCFC) commercially since the 1930s has been chloro- difluoromethane, or HCFC-22, which has the undesirable The consumer appetite for safe household refrigeration led effect of depleting stratospheric ozone. As this molecule to the commercialisation in the 1930s of dichlorodifluo- is currently being phased out under the Montreal Pro- romethane, or CFC-12, a non-flammable and non-toxic re- tocol, monitoring its concentration profiles using infrared frigerant (Myers, 2007). Within the next few decades, other sounders crucially requires accurate laboratory spectroscopic chemically related refrigerants were additionally commer- data. This work describes new high-resolution infrared ab- cialised, including chlorodifluoromethane, or a hydrochlo- sorption cross sections of chlorodifluoromethane over the rofluorocarbon known as HCFC-22, which found use in a spectral range 730–1380 cm−1, determined from spectra wide array of applications such as air conditioners, chillers, recorded using a high-resolution Fourier transform spectrom- and refrigeration for food retail and industrial processes. -

SAFETY DATA SHEET Halocarbon R-503
SAFETY DATA SHEET Halocarbon R-503 Section 1. Identification GHS product identifier : Halocarbon R-503 Other means of : Not available. identification Product type : Liquefied gas Product use : Synthetic/Analytical chemistry. SDS # : 007306 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Liquefied gas substance or mixture HAZARDOUS TO THE OZONE LAYER - Category 1 GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May cause frostbite. May displace oxygen and cause rapid suffocation. Harms public health and the environment by destroying ozone in the upper atmosphere. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. Use only equipment of compatible materials of construction. Always keep container in upright position. Prevention : Not applicable. Response : Not applicable. Storage : Protect from sunlight. Store in a well-ventilated place. Disposal : Refer to manufacturer or supplier for information on recovery or recycling. Hazards not otherwise : Liquid can cause burns similar to frostbite. -

SAFETY DATA SHEET Halocarbon R-23 (Trifluoromethane)
SAFETY DATA SHEET Halocarbon R-23 (Trifluoromethane) Section 1. Identification GHS product identifier : Halocarbon R-23 (Trifluoromethane) Chemical name : trifluoromethane Other means of : Fluoroform; Arcton 1; Fluoryl; Freon F-23; Freon 23; Genetron 23; Methyl trifluoride; R identification 23; Trifluoromethane; CHF3; Arcton; Halocarbon 23; UN 1984; Carbon trifluoride; Genetron HFC23; Propellant 23; Refrigerant 23 Product use : Synthetic/Analytical chemistry. Synonym : Fluoroform; Arcton 1; Fluoryl; Freon F-23; Freon 23; Genetron 23; Methyl trifluoride; R 23; Trifluoromethane; CHF3; Arcton; Halocarbon 23; UN 1984; Carbon trifluoride; Genetron HFC23; Propellant 23; Refrigerant 23 SDS # : 000023 Supplier's details : ASPEN Refrigerants, Inc. 38-18 33rd Street Long Island City, NY 11101 1-800-473-3766 Emergency telephone :1-800-424-9300 number (with 24 hours of operation) Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Liquefied gas substance or mixture GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May cause frostbite. May displace oxygen and cause rapid suffocation. Precautionary statements General : Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Prevention : Use and store only outdoors or in a well-ventilated place. Response : Not applicable. Storage : Protect from sunlight. Store in a well-ventilated place. Disposal : Not applicable. Hazards not otherwise : Liquid can cause burns similar to frostbite. May displace oxygen and cause rapid classified suffocation. Date of issue/Date of revision 2/1/2019 Date of previous issue : No previous validation. -

Utilization of Fluoroform for Difluoromethylation in Continuous
Green Chemistry View Article Online COMMUNICATION View Journal | View Issue Utilization of fluoroform for difluoromethylation in continuous flow: a concise synthesis of Cite this: Green Chem., 2018, 20, 108 α-difluoromethyl-amino acids† Received 26th September 2017, Accepted 30th October 2017 Manuel Köckinger,a Tanja Ciaglia,a Michael Bersier,b Paul Hanselmann,b DOI: 10.1039/c7gc02913f Bernhard Gutmann *a,c and C. Oliver Kappe *a,c rsc.li/greenchem Fluoroform (CHF3) can be considered as an ideal reagent for trolled under the Montreal Protocol. As a consequence, its pro- difluoromethylation reactions. However, due to the low reactivity duction and usage has become increasingly limited and expen- of fluoroform, only very few applications have been reported so sive. A plethora of alternative difluoromethane sources have far. Herein we report a continuous flow difluoromethylation proto- been developed in recent years, including TMSCF2Br, 3 2 col on sp carbons employing fluoroform as a reagent. The proto- (EtO)2POCF2Br, PhCOCF2Cl and CHF2OTf. Although these col is applicable for the direct Cα-difluoromethylation of protected reagents cover the needs of chemists for difluoromethylation Creative Commons Attribution 3.0 Unported Licence. α-amino acids, and enables a highly atom efficient synthesis of the on a laboratory scale, their high cost, low atom economy and active pharmaceutical ingredient eflornithine. limited commercial availability prohibit their usage in an industrial setting. The difluoromethyl group is found in an increasing array of The most attractive CF - and CHF -source is fluoroform 1 3 2 pharmaceutical and agrochemical products. Not surprisingly, (CHF , Freon 23). Fluoroform is generated as a large-volume ff 3 therefore, significant e orts have been devoted towards the waste-product during the synthesis of chlorodifluoromethane development of novel protocols for the introduction of the (Fig. -

Direct Nucleophilic Trifluoromethylation of Carbonyl Compounds By
www.nature.com/scientificreports OPEN Direct nucleophilic trifuoromethylation of carbonyl compounds by potent greenhouse Received: 16 April 2018 Accepted: 18 July 2018 gas, fuoroform: Improving Published: xx xx xxxx the reactivity of anionoid trifuoromethyl species in glymes Takuya Saito1, Jiandong Wang1, Etsuko Tokunaga1, Seiji Tsuzuki2 & Norio Shibata 1,3 A simple protocol to overcome the problematic trifuoromethylation of carbonyl compounds by the potent greenhouse gas “HFC-23, fuoroform” with a potassium base is described. Simply the use of glymes as a solvent or an additive dramatically improves the yields of this transformation. Experimental results and DFT calculations suggest that the benefcial efect deals with glyme coordination to the + + K to produce [K(polyether)n] whose diminished Lewis acidity renders the reactive anionoid CF3 − counterion species more ‘naked’, thereby slowing down its undesirable decomposition to CF2 and F and simultaneously increasing its reactivity towards the organic substrate. Tere has been remarkable progress recently in the synthetic incorporation of a trifuoromethyl (CF3) moiety into potential bioactive molecules, prompting the discovery of new pharmaceuticals and agrochemicals1–5. Fluoroform (HFC-23, HCF3, trifuoromethane) is a potent greenhouse gas that is formed as a by-product in huge amounts during the synthesis of poly-tetrafuoroethylene (PTFE) and polyvinylidene difuoride (PVDF) from chlorodifuoromethane (ClCHF2). Fluoroform has a 11,700-fold higher GWP than carbon dioxide with an atmos- pheric lifetime of 264 years and is used to a very limited extent as a refrigerant or as a raw material6–10. At present, fuoroform abatement techniques involve thermal oxidation, catalytic hydrolysis and plasma destruction, so there are operation and economical limits to transform fuoroform to useful refrigerants or fre extinguishers11–17. -

III IIII US005523499A United States Patent (19) 11 Patent Number: 5,523,499 Corbin Et Al
III IIII US005523499A United States Patent (19) 11 Patent Number: 5,523,499 Corbin et al. (45) Date of Patent: Jun. 4, 1996 54 PURIFICATION OF HEXAFLUOROETHANE 4,940,825 7/1990 Yates ....................................... 570/179 PRODUCTS 4,950,816 8/1990 Tung et al. ............................. 570/79 75 Inventors: David R. Corbin, West Chester, Pa.; FOREIGN PATENT DOCUMENTS Richard E. Fernandez, Bear, Del.: 0389334 9/1990 European Pat. Off.. Barry A. Mahler, Glen Mills, Pa. 3311751 10/1984 Germany . 3-72437 3/1991 Japan. 73 Assignee: E. I. Du Pont de Nemours and WO90/O8751 8/1990 WIPO. Company, Wilmington, Del. OTHER PUBLICATIONS 21 Appl. No.: 295,669 Glajch, J. L. et al., "Column Packings for On-Line GC 22, PCT Filed: Mar. 10, 1992 Analysis of Fluorocarbons in the Presence of Reactive Gases", LC-GC, 4(6), 574-577 (1986). 86 PCT No.: PCT/US92/O1607 Woytek, A. J., “Appendix 11.11 The role of fluorocarbon 371 Date: Dec. 28, 1994 gases in the microelectronics industry', J. Fluor: Chem., 33, S e ec. Zas, 331-334 (1986). S 102(e) Date: Dec. 28, 1994 Szostak, R., Molecular Sieves: Principles of Synthesis and (87) PCT Pub. No.: WO93/17988 Identification, pp. 2-6, Van Nostrand Reinhold, New York u (1989). PCT Pub.. Date: SepSep. 16, 1993 Primary Examiner-Alan Siegel I51) Int. Cl." ............................................ CO7C 17/38 (52 U.S. C. ............................................. 570/179; 570/164 (57) ABSTRACT 58 Field of Search ...................................... 570/179, 164 A process is disclosed for purifying a hexafluoroethane product containing CClF and/or CHF impurities which 56 References Cited comprises the step of contacting the product with a sorbent U.S. -

Section 2. Hazards Identification OSHA/HCS Status : This Material Is Considered Hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200)
SAFETY DATA SHEET Halocarbon R-508A Section 1. Identification GHS product identifier : Halocarbon R-508A Other means of : Blend of Hexafluoroethane/Trifluoromethane; R116/R23, PFC116/HFC23 identification Product type : Gas. Product use : Synthetic/Analytical chemistry. Synonym : Blend of Hexafluoroethane/Trifluoromethane; R116/R23, PFC116/HFC23 SDS # : 008070 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Compressed gas substance or mixture GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May displace oxygen and cause rapid suffocation. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. Use only equipment of compatible materials of construction. Prevention : Not applicable. Response : Not applicable. Storage : Protect from sunlight. Store in a well-ventilated place. Disposal : Not applicable. Hazards not otherwise : In addition to any other important health or physical hazards, this product may displace classified oxygen and cause rapid suffocation. Date of issue/Date of revision : 8/17/2018 Date of previous issue : No previous validation Version : 1 1/11 Halocarbon R-508A Section 3. -

Haloform Reaction - Wikipedia
6/13/2020 Haloform reaction - Wikipedia Haloform reaction The haloform reaction is a chemical reaction where a haloform Haloform reaction (CHX , where X is a halogen) is produced by the exhaustive 3 Named after Adolf Lieben halogenation of a methyl ketone (RCOCH3, where R can be either a hydrogen atom, an alkyl or an aryl group), in the presence of a Reaction type Substitution base.[1][2][3] The reaction can be used to transform acetyl groups into reaction carboxyl groups or to produce chloroform (CHCl3), bromoform Identifiers (CHBr3), or iodoform (CHI3) and also cyanide. Organic haloform-reaction Chemistry Portal Contents Mechanism Scope Applications Laboratory scale Industrially As a by-product of water chlorination History References Mechanism In the first step, the halogen disproportionates in the presence of hydroxide to give the halide and hypohalite (example with bromine, but reaction is the same in case of chlorine and iodine; one should only substitute Br for Cl or I): If a secondary alcohol is present, it is oxidized to a ketone by the hypohalite: If a methyl ketone is present, it reacts with the hypohalite in a three-step process: 1. Under basic conditions, the ketone undergoes keto-enol tautomerization. The enolate undergoes electrophilic attack by the hypohalite (containing a halogen with a formal +1 charge). https://en.wikipedia.org/wiki/Haloform_reaction#Iodoform_reaction 1/5 6/13/2020 Haloform reaction - Wikipedia 2. When the α(alpha) position has been exhaustively halogenated, the molecule undergoes a nucleophilic − acyl substitution by hydroxide, with CX3 being the leaving group stabilized by three electron- − withdrawing groups. -
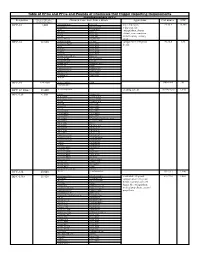
Table of Hfcs and Pfcs and Pounds of Chemicals That Trigger
Table of HFCs and PFCs and Pounds of Chemicals that Trigger Reporting Requirements Hydrofluorocarbons (HFCs) Designation Pounds of Substance = Chemical Name, Trade Names, Blends Applications CAS number GWP 10,000 metric tons Ce HFC-23 1,480 trifluoromethane Suva 95 low temperature 75-46-7 14,800 fluoroform Genetron 503 refrigerant, fire R-23 Genetron 23 extinguishant, plasma Freon 23 Klea 23 FE-13 Klea 508 etchant, semi-conductor FE-36 Klea 5R3 manufacturing cleaning Forane FX 220 NARM 503 agent HFC-32 32,660 difluoromethane R-407C component in refrigerant 75-10-5 675 methylene fluoride Klea 407A blends R-32 Klea 407B R-410A Klea 407C R-407C Klea 407D Freon 32 Klea 410A Forane 410A (AZ-20) Klea 32 Forane FX 40 Genetron 407C Forane FX 220 AZ-20 Forane 407C EcoloAce 407c Forane 32 HX4 Asahiklin SA-39 Solkane 407C Asahiklin SA-45 Solkane 410 Meforex 98 Suva 9000 Meforex 32 Suva 9100 R-410A HFC-41 227,280 methyl fluoride R-41 593-53-3 97 fluoromethane HFC-43-10mee 13,440 decafluoropentane cleaning solvent 138495-42-8 1,640 HFC-125 6,300 ethane Forane 507 pentafluoro Forane FX10 pentafluoroethane Forane FX40 R-125 Forane FX70 FC-125 Suva 125 Freon 125 Suva 9000 FE-25 Suva 9100 Arcton 402A Suva HP62 Arcton 402B Suva HP80 Arcton 408A Suva HP81 Klea 404A Cooltop R-134a Replacement Klea 407A EcoloAce 404a Klea 407B Di 44 Klea 407C Meforex 125 Klea 407D Meforex 55 Klea 410A Meforex 57 Klea 507A Meforex 98 Asahiklin SA-28 ISCEON 29 Asahiklin SA-39 ISCEON 404A Asahiklin SA-45 ISCEON 507 AZ-20 ISCEON 59 AZ-50 ISCEON 79 Genetron 404A ISCEON 89 Genetron -

HCFC-22) 9 10 11 by 12 13 14 15 Jeremy J
Atmos. Meas. Tech. Discuss., doi:10.5194/amt-2015-389, 2016 Manuscript under review for journal Atmos. Meas. Tech. Published: 18 January 2016 c Author(s) 2016. CC-BY 3.0 License. 1 13 January 2016 2 3 4 5 6 7 New and improved infrared absorption cross sections for 8 chlorodifluoromethane (HCFC-22) 9 10 11 by 12 13 14 15 Jeremy J. Harrison1,2 16 17 1Department of Physics and Astronomy, University of Leicester, University Road, Leicester 18 LE1 7RH, United Kingdom 19 2National Centre for Earth Observation, University of Leicester, University Road, Leicester 20 LE1 7RH, United Kingdom 21 22 23 Number of pages = 19 24 Number of tables = 2 25 Number of figures = 4 26 27 28 29 Address for correspondence: 30 31 Dr. Jeremy J. Harrison 32 Department of Physics and Astronomy 33 University of Leicester 34 University Road 35 Leicester LE1 7RH 36 United Kingdom 37 38 e-mail: [email protected] 39 Atmos. Meas. Tech. Discuss., doi:10.5194/amt-2015-389, 2016 Manuscript under review for journal Atmos. Meas. Tech. Published: 18 January 2016 c Author(s) 2016. CC-BY 3.0 License. 40 Abstract 41 The most widely used hydrochlorofluorocarbon (HCFC) commercially since the 42 1930s has been chlorodifluoromethane, or HCFC-22, which has the undesirable effect of 43 depleting stratospheric ozone. As this molecule is currently being phased out under the 44 Montreal Protocol, monitoring its concentration profiles using infrared sounders crucially 45 requires accurate laboratory spectroscopic data. This work describes new high-resolution 46 infrared absorption cross sections of chlorodifluoromethane over the spectral range 730 – 47 1380 cm-1, determined from spectra recorded using a high-resolution Fourier transform 48 spectrometer (Bruker IFS 125HR) and a 26-cm-pathlength cell.