Reaction of Potassium Fluoride with Organic Halogen Compounds. I
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
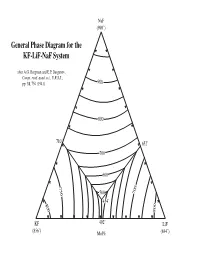
General Phase Diagram for the KF-Lif-Naf System
NaF (990˚) General Phase Diagram for the KF-LiF-NaF System after A.G. Bergman and E.P. Dergunov, Compt. rend. acad. sci., U.R.S.S., pp. 31, 754 (1941). 900 800 710˚ 652˚ 700 600 750 500 700 454˚ 800 800 KF 492˚ LiF (856˚) Mol% (844˚) Sodium Fluoride NaF 950˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 940˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 930˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 920˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 910˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 900˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 890˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 880˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 870˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 860˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 850˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 840˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 830˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 820˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 810˚ 810˚ ˚ KF 810 LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 800˚ 800˚ ˚ KF 800 LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 790˚ 790˚ ˚ KF 790 LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 780˚ 780˚ ˚ LiF KF 780 Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 770˚ 770˚ ˚ LiF KF 770 Potassium Fluoride -

United States Patent Office Patented Jan
2,732,410 United States Patent Office Patented Jan. 24, 1956 2 The process can be carried out in various ways. Thus, 2,732,410 the vaporized halogen fluoride can be passed, if desired PROCESS FOR PREPARNG TETRAFLUORO with an inert carrier gas such as nitrogen, argon or helium, ETHYLENE BY REACTING CARBON AND through a column of carbon heated at a temperature of A BINARY HALOGEN FLUORDE at least 1500 C. in a suitable reactor, e. g., a graphite Mark W. Farlow, Holly Oak, and Earl L. Muetterties, tube placed inside a resistance furnace or an induction Hockessin, Dei, assignors to E. I. du Post de Neois's furnace. The gaseous reaction products are then imme and Company, Wilmington, Del, a corporation of Dela diately treated, as described below, to remove any un Ware reacted halogen fluoride and any free chlorine or bromine, 0 in order to minimize or eliminate the possibility of their No Drawing. Application January 12, 1955, reacting with the tetrafluoroethylene present in the re Serial No. 481,482 action product. A preferred mode of operation consists in 4 Claims. (C. 260-653) reacting the halogen fluoride with the carbon electrodes of a carbon arc, where the temperature is estimated to be in This invention relates to a new process of preparing 15 the range of 2500 to 3500-4000 C., and again immediate compounds containing only carbon and fluorine, or car ly removing from the effluent gas any halogen fluoride and bon, fluorine and another halogen, such compounds be free reactive halogen which may be present. -

Sulfur Hexafluoride Hazard Summary Identification
Common Name: SULFUR HEXAFLUORIDE CAS Number: 2551-62-4 RTK Substance number: 1760 DOT Number: UN 1080 Date: April 2002 ------------------------------------------------------------------------- ------------------------------------------------------------------------- HAZARD SUMMARY * Sulfur Hexafluoride can affect you when breathed in. * If you think you are experiencing any work-related health * Sulfur Hexafluoride can irritate the skin causing a rash or problems, see a doctor trained to recognize occupational burning feeling on contact. Direct skin contact can cause diseases. Take this Fact Sheet with you. frostbite. * Exposure to hazardous substances should be routinely * Sulfur Hexafluoride may cause severe eye burns leading evaluated. This may include collecting personal and area to permanent damage. air samples. You can obtain copies of sampling results * Breathing Sulfur Hexafluoride can irritate the nose and from your employer. You have a legal right to this throat. information under OSHA 1910.1020. * Breathing Sulfur Hexafluoride may irritate the lungs causing coughing and/or shortness of breath. Higher WORKPLACE EXPOSURE LIMITS exposures can cause a build-up of fluid in the lungs OSHA: The legal airborne permissible exposure limit (pulmonary edema), a medical emergency, with severe (PEL) is 1,000 ppm averaged over an 8-hour shortness of breath. workshift. * High exposure can cause headache, confusion, dizziness, suffocation, fainting, seizures and coma. NIOSH: The recommended airborne exposure limit is * Sulfur Hexafluoride may damage the liver and kidneys. 1,000 ppm averaged over a 10-hour workshift. * Repeated high exposure can cause deposits of Fluorides in the bones and teeth, a condition called “Fluorosis.” This ACGIH: The recommended airborne exposure limit is may cause pain, disability and mottling of the teeth. -

United States Patent Office Patented July 1, 1969
3,453,337 United States Patent Office Patented July 1, 1969 1. 2 3,453,337 FLUORINATION OF HALOGENATED The presence in the reaction mixture of the two fluo ORGANIC COMPOUNDS rides, or the complex fluoride enables better yields of Royston Henry Bennett and David Walter Cottrell, Ayon highly fluorinated products to be obtained under less mouth, England, assignors to Imperial Smelting Cor severe reaction condions, markedly increases the amount poration (N.S.C.) Limited, London, England, a British of fluorination reagent reacted under otherwise similar company conditions and enables the fluorination reaction to be No brawing. Filed Feb. 19, 1965, Ser. No. 434,128 carried out (for the same yield of product) at a lower Claims priority, application Great Britain, Feb. 26, 1964, temperature with the consequent use of less costly ma 7,932/64 terials and techniques of reactor construction. The pres Int, C. C07c 25/04 10 ence of the fluorides enables the vapor phase reaction U.S. C. 260-650 2 Claims to be carried out (for the same yields) at lower pressure This invention relates to the fluorination of organic than the pressure involved in the reactions using only halogen compounds and more especially to a process the alkali metal fluorides as proposed hitherto. The fur for the production of highly fluorinated aromatic com ther possibility of using a continuous flow apparatus such pounds by the replacement of higher halogen atoms in 15 as a fluidised reactor will be apparent to those familiar halogeno-aromatic compounds by fluorine atoms. with the art. The presence of the two fluorides or com Aromatic halogenocarbons containing carbon and halo plex fluoride enables a lower temperature to be em gen atoms only can be reacted with alkali fluorides ployed than was hitherto believed to be necessary, with under various conditions to give yields of halofluoro a consequent reduction in the extent of thermal degrada aromatic compounds. -

Effects of Fluoride and Other Halogen Ions on the External Stress Corrosion Cracking of Type 304 Austenitic Stainless Steel
NUREG/CR-6539 _ Effects of Fluoride and Other Halogen Ions on the External Stress Corrosion Cracking of Type 304 Austenitic Stainless Steel M low, F. B. Ilutto, Jr. Tutco Scientific Corporation Prepared for U.S. Nuclear Regulatory Commission pfo)D'' ~%, 1 a- ! '% .. lill|Il||# lilllll:[ll[Ill]Ol|Ill E M 72A8A!8 * 7 * CR-6539 R PDR __ . - . .-- I | ! ' AVAILABILITY NOTICE Availabilny of Reference Matenals Cited in NRC Pubhcations Most documents cited in NRC publications will be available from one of the following sources: 1. The NRC Public Document Room, 2120 L Street, NW., Lower Level. Washington, DC 20555-0001 i | 2. The Superintendent of Documents, U.S. Government Pnnting Office, P. O. Box 37082. Washington, DC 20402-9328 l 3. The National Technical information Service Springfield, VA 22161-0002 Although the listing that follows represents the majority of documents cited in NRC publications, it is not in- tended to be exhaustive. Referenced documents available for inspection and copylng for a fee from the NRC Public Document Room include NRC correspondence and internal NRC memoranda; NRC bulletins, circulars, information notices, in- spection and investigation notices; licensee event reports; vendor reports and correspondence; Commissen papers; and applicant and licensee documents and correspondence. The following documents in the NUREG series are aves slo for purchase from the Government Printing Office: formal NRC staff and contractor reports, NRC-sponsored conference proceedings, internatior,al agreement reports, grantee reports, and NRC booklets and brochures. Also available are regulatory guides NRC regula- tions in the Code of Federal Regulations, and Nuclear Regulatory Commission issuances. -

Ammonium Fluoride Product Stewardship Summary February 2012
Ammonium Fluoride Product Stewardship Summary February 2012 (NH4)F Chemical Name: Ammonium Fluoride Chemical Category (if applicable): Inorganic neutral halide Synonyms: Neutral ammonium fluoride; Commercial ammonium fluoride; and (NH4)F CAS Number: 12125-01-8 CAS Name: Ammonium fluoride EC (EINECS) Number: 235-185-9 Other identifier (Please specify): GPS0050 Honeywell manufactures ammonium fluoride that is used by industry in the manufacture of electronic materials. Exposure can occur at either an ammonium fluoride manufacturing facility or at other manufacturing, packaging or storage facilities that handle ammonium fluoride. Persons involved in maintenance, sampling and testing activities, or in the loading and unloading of packages containing ammonium fluoride are at risk of exposure, but worker exposure can be controlled with the use of proper general mechanical ventilation and personal protective equipment. Workplace exposure limits for fluoride ion have been established for use in worksite safety programs. When ammonium fluoride is a component of consumer products, users should follow manufacturer’s use and/or label instructions. Ammonium fluoride dusts released to the atmosphere and deposited in soil or surface water in the vicinity of production sites have negligible impact on the environment. Please see the MSDS for additional information. Ammonium fluoride is a nonflammable solid that is stable under normal conditions. Ammonium fluoride is corrosive to metals and glass. It can react with acids to liberate hydrogen fluoride and bases to liberate ammonia. When heated to decomposition, it will release toxic hydrogen fluoride gas and ammonia. Contact of ammonium fluoride with water or extended skin contact under moist conditions can produce hydrofluoric acid (HF), a very dangerous acid that can cause burns of the skin and eyes. -

ADA Fluoridation Facts 2018
Fluoridation Facts Dedication This 2018 edition of Fluoridation Facts is dedicated to Dr. Ernest Newbrun, respected researcher, esteemed educator, inspiring mentor and tireless advocate for community water fluoridation. About Fluoridation Facts Fluoridation Facts contains answers to frequently asked questions regarding community water fluoridation. A number of these questions are responses to myths and misconceptions advanced by a small faction opposed to water fluoridation. The answers to the questions that appear in Fluoridation Facts are based on generally accepted, peer-reviewed, scientific evidence. They are offered to assist policy makers and the general public in making informed decisions. The answers are supported by over 400 credible scientific articles, as referenced within the document. It is hoped that decision makers will make sound choices based on this body of generally accepted, peer-reviewed science. Acknowledgments This publication was developed by the National Fluoridation Advisory Committee (NFAC) of the American Dental Association (ADA) Council on Advocacy for Access and Prevention (CAAP). NFAC members participating in the development of the publication included Valerie Peckosh, DMD, chair; Robert Crawford, DDS; Jay Kumar, DDS, MPH; Steven Levy, DDS, MPH; E. Angeles Martinez Mier, DDS, MSD, PhD; Howard Pollick, BDS, MPH; Brittany Seymour, DDS, MPH and Leon Stanislav, DDS. Principal CAAP staff contributions to this edition of Fluoridation Facts were made by: Jane S. McGinley, RDH, MBA, Manager, Fluoridation and Preventive Health Activities; Sharon (Sharee) R. Clough, RDH, MS Ed Manager, Preventive Health Activities and Carlos Jones, Coordinator, Action for Dental Health. Other significant staff contributors included Paul O’Connor, Senior Legislative Liaison, Department of State Government Affairs. -

2. Relevance to Public Health
FLUORIDES, HYDROGEN FLUORIDE, AND FLUORINE 15 2. RELEVANCE TO PUBLIC HEALTH 2.1 BACKGROUND AND ENVIRONMENTAL EXPOSURES TO FLUORIDES, HYDROGEN FLUORIDE, AND FLUORINE IN THE UNITED STATES Fluorine is the most electronegative and reactive of all elements; fluoride is the ionic form of fluorine. Fluorine and anhydrous hydrogen fluoride are naturally occurring gases that have a variety of industrial uses including the production of fluorine-containing chemicals, pharmaceuticals, high octane gasoline, and fluorescent light bulbs; aqueous hydrofluoric acid is a liquid used for stainless steel pickling, glass etching, and metal coatings. The general population is typically exposed to very low levels of gaseous fluoride (primarily as hydrogen fluoride); in the United States and Canada, the levels ranged from 0.01 to 1.65 µg/m3. Populations living near industrial sources of hydrogen fluoride, including coal burning facilities, may be exposed to higher levels of hydrogen fluoride in the air. Additionally, vegetables and fruits grown near these sources may contain higher levels of fluoride, particularly from fluoride- containing dust settling on the plants. Fluoride salts, generically referred to as fluorides, are naturally occurring components of rocks and soil. One of the more commonly used fluoride salt is sodium fluoride; its principal use is for the prevention of dental caries. Sodium fluoride and other fluoride compounds, such as fluorosilicic acid and sodium hexafluorisilicate, are used in the fluoridation of public water. Sodium monofluorophosphate and stanneous fluoride are commonly used in dentifrices such as toothpaste. The general population can be exposed to fluoride through the consumption of fluoridated drinking water, food, and dentifrices. -

HYDROGEN FLUORIDE Safety Data Sheet
Revision Date 14-May-2015 , Version 1 _________________________________________________________________________________ HYDROGEN FLUORIDE Safety Data Sheet _________________________________________________________________________________ 1. IDENTIFICATION Product identifier Product Name HYDROGEN FLUORIDE Other means of identification Safety data sheet number LIND-P070 UN/ID no. UN1052 Synonyms Hydrofluoric acid, anhydrous Recommended use of the chemical and restrictions on use Recommended Use Industrial and professional use. Uses advised against Consumer use Details of the supplier of the safety data sheet Linde Gas North America LLC - Linde Merchant Production Inc. - Linde LLC 575 Mountain Ave. Murray Hill, NJ 07974 Phone: 908-464-8100 www.lindeus.com Linde Gas Puerto Rico, Inc. Road 869, Km 1.8 Barrio Palmas, Catano, PR 00962 Phone: 787-641-7445 www.pr.lindegas.com Linde Canada Limited 5860 Chedworth Way Mississauga, Ontario L5R 0A2 Phone: 905-501-1700 www.lindecanada.com * May include subsidiaries or affiliate companies/divisions. For additional product information contact your local customer service. Emergency telephone number Company Phone Number 800-232-4726 (Linde National Operations Center, US) 905-501-0802 (Canada) CHEMTREC: 1-800-424-9300 (North America) +1-703-527-3887 (International) 2. HAZARDS IDENTIFICATION _____________________________________________________________________________________________ Page 1 / 11 LIND-P070 HYDROGEN FLUORIDE Revision Date 14-May-2015 _____________________________________________________________________________________________ -

Fluorides, Hydrogen Fluoride, and Fluorine Cas # 7681-49-4, 7664-39-3, 7782-41-4
FLUORIDES, HYDROGEN FLUORIDE, AND FLUORINE CAS # 7681-49-4, 7664-39-3, 7782-41-4 Division of Toxicology ToxFAQsTM September 2003 This fact sheet answers the most frequently asked health questions (FAQs) about fluorides, hydrogen fluoride, and fluorine. For more information, call the ATSDR Information Center at 1-888-422-8737. This fact sheet is one in a series of summaries about hazardous substances and their health effects. It is important you understand this information because these substances may harm you. The effects of exposure to any hazardous substance depend on the dose, the duration, how you are exposed, personal traits and habits, and whether other chemicals are present. HIGHLIGHTS: Fluorides are naturally occurring compounds. Low levels of fluorides can help prevent dental cavities. At high levels, fluorides can result in tooth and bone damage. Hydrogen fluoride and fluorine are naturally-occurring gases that are very irritating to the skin, eyes, and respiratory tract. These substances have been found in at least 188 of the 1,636 National Priorities List sites identified by the Environmental Protection Agency (EPA). What are fluorides, hydrogen fluoride, and are carried by wind and rain to nearby water, soil, and food fluorine? sources. Fluorides, hydrogen fluoride, and fluorine are chemically ‘Fluorides in water and soil will form strong associations related. Fluorine is a naturally-occurring, pale yellow-green with sediment or soil particles. gas with a sharp odor. It combines with metals to make ‘Fluorides will accumulate in plants and animals. In fluorides such as sodium fluoride and calcium fluoride, both animals, the fluoride accumulates primarily in the bones or white solids. -

Fluoride Fact Sheet
International Headquarters & Laboratory Phone 630 505 0160 WWW.WQA.ORG A not-for-profit organization FLUORIDE FACT SHEET Contaminant In Water As Maximum Contaminant Level US EPA: - - Fluoride (F ) Fluoride ion, F MCL* = 4.0 mg/L or ppm Secondary Standard** = 2.0 mg/L or ppm WHO† Guideline = 1.5mg/L Natural deposits Sources of Contaminant Municipally treated drinking water (> 2 mg/L, potentially as a result of poorly monitored or malfunctioning feeding equipment) Skeletal fluorosis, from long-term consumption at > 4 mg/L (a Potential Health Effects serious bone disorder resembling osteopetrosis and characterized by extreme density and hardness and abnormal fragility of the bones) Mottling (discoloration) of teeth in children under 9 years of age Potential Aesthetic Effects (from long-term consumption at > 2 mg/L) Disfiguration/pitting of teeth in children Treatment Methods Reverse Osmosis Strong base anion exchange (Cl- form) Point-of-Entry Activated alumina adsorption media Point-of-Use Distillation *Maximum Contaminant Level (MCL) - The highest level of a contaminant that is allowed in drinking water. MCLs are set as close to MCLGs as feasible using the best available treatment technology and taking cost into consideration. MCLs are enforceable standards. **National Secondary Drinking Water Regulations (NSDWRs or secondary standards) are non-enforceable guidelines regulating contaminants that may cause cosmetic effects (such as skin or tooth discoloration) or aesthetic effects (such as taste, odor, or color) in drinking water. EPA recommends secondary standards to water systems but does not require systems to comply. However, states may choose to adopt them as enforceable standards. WHO† - World Health Organization Fluorine is a natural trace element and exists in almost all soils. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride