United States Patent Office E 8
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
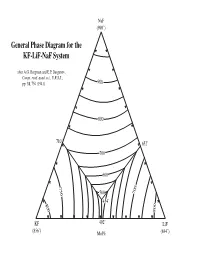
General Phase Diagram for the KF-Lif-Naf System
NaF (990˚) General Phase Diagram for the KF-LiF-NaF System after A.G. Bergman and E.P. Dergunov, Compt. rend. acad. sci., U.R.S.S., pp. 31, 754 (1941). 900 800 710˚ 652˚ 700 600 750 500 700 454˚ 800 800 KF 492˚ LiF (856˚) Mol% (844˚) Sodium Fluoride NaF 950˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 940˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 930˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 920˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 910˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 900˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 890˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 880˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 870˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 860˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 850˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 840˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 830˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 820˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 810˚ 810˚ ˚ KF 810 LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 800˚ 800˚ ˚ KF 800 LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 790˚ 790˚ ˚ KF 790 LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 780˚ 780˚ ˚ LiF KF 780 Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 770˚ 770˚ ˚ LiF KF 770 Potassium Fluoride -

United States Patent Office Patented July 1, 1969
3,453,337 United States Patent Office Patented July 1, 1969 1. 2 3,453,337 FLUORINATION OF HALOGENATED The presence in the reaction mixture of the two fluo ORGANIC COMPOUNDS rides, or the complex fluoride enables better yields of Royston Henry Bennett and David Walter Cottrell, Ayon highly fluorinated products to be obtained under less mouth, England, assignors to Imperial Smelting Cor severe reaction condions, markedly increases the amount poration (N.S.C.) Limited, London, England, a British of fluorination reagent reacted under otherwise similar company conditions and enables the fluorination reaction to be No brawing. Filed Feb. 19, 1965, Ser. No. 434,128 carried out (for the same yield of product) at a lower Claims priority, application Great Britain, Feb. 26, 1964, temperature with the consequent use of less costly ma 7,932/64 terials and techniques of reactor construction. The pres Int, C. C07c 25/04 10 ence of the fluorides enables the vapor phase reaction U.S. C. 260-650 2 Claims to be carried out (for the same yields) at lower pressure This invention relates to the fluorination of organic than the pressure involved in the reactions using only halogen compounds and more especially to a process the alkali metal fluorides as proposed hitherto. The fur for the production of highly fluorinated aromatic com ther possibility of using a continuous flow apparatus such pounds by the replacement of higher halogen atoms in 15 as a fluidised reactor will be apparent to those familiar halogeno-aromatic compounds by fluorine atoms. with the art. The presence of the two fluorides or com Aromatic halogenocarbons containing carbon and halo plex fluoride enables a lower temperature to be em gen atoms only can be reacted with alkali fluorides ployed than was hitherto believed to be necessary, with under various conditions to give yields of halofluoro a consequent reduction in the extent of thermal degrada aromatic compounds. -

Halide Metathesis in Overdrive: Mechanochemical Synthesis of a Heterometallic Group 1 Allyl Complex
Halide metathesis in overdrive: mechanochemical synthesis of a heterometallic group 1 allyl complex Ross F. Koby1, Nicholas R. Rightmire1, Nathan D. Schley1, Timothy P. Hanusa*1 and William W. Brennessel2 Full Research Paper Open Access Address: Beilstein J. Org. Chem. 2019, 15, 1856–1863. 1Department of Chemistry, Vanderbilt University, PO Box 1822, doi:10.3762/bjoc.15.181 Nashville, TN 37235, USA and 2X-ray Crystallographic Facility, B51 Hutchison Hall, Department of Chemistry, University of Rochester, Received: 01 March 2019 Rochester, NY 14627, USA Accepted: 18 July 2019 Published: 02 August 2019 Email: Timothy P. Hanusa* - [email protected] This article is part of the thematic issue "Mechanochemistry II". * Corresponding author Guest Editor: J. G. Hernández Keywords: © 2019 Koby et al.; licensee Beilstein-Institut. caesium; entropy; intermolecular forces; mechanochemistry; License and terms: see end of document. metathesis; potassium Abstract As a synthesis technique, halide metathesis (n RM + M'Xn → RnM' + n MX) normally relies for its effectiveness on the favorable formation of a metal halide byproduct (MX), often aided by solubility equilibria in solution. Owing to the lack of significant thermodynamic driving forces, intra-alkali metal exchange is one of the most challenging metathetical exchanges to attempt, espe- cially when conducted without solvent. Nevertheless, grinding together the bulky potassium allyl [KA']∞ (A' = [1,3- – (SiMe3)2C3H3] ) and CsI produces the heterometallic complex [CsKA'2]∞ in low yield, which was crystallographically character- ized as a coordination polymer that displays site disorder of the K+ and Cs+ ions. The entropic benefits of mixed Cs/K metal … … centers, but more importantly, the generation of multiple intermolecular K CH3 and Cs CH3 interactions in [CsKA'2]∞, enable an otherwise unfavorable halide metathesis to proceed with mechanochemical assistance. -

Periodic Trends and the S-Block Elements”, Chapter 21 from the Book Principles of General Chemistry (Index.Html) (V
This is “Periodic Trends and the s-Block Elements”, chapter 21 from the book Principles of General Chemistry (index.html) (v. 1.0M). This book is licensed under a Creative Commons by-nc-sa 3.0 (http://creativecommons.org/licenses/by-nc-sa/ 3.0/) license. See the license for more details, but that basically means you can share this book as long as you credit the author (but see below), don't make money from it, and do make it available to everyone else under the same terms. This content was accessible as of December 29, 2012, and it was downloaded then by Andy Schmitz (http://lardbucket.org) in an effort to preserve the availability of this book. Normally, the author and publisher would be credited here. However, the publisher has asked for the customary Creative Commons attribution to the original publisher, authors, title, and book URI to be removed. Additionally, per the publisher's request, their name has been removed in some passages. More information is available on this project's attribution page (http://2012books.lardbucket.org/attribution.html?utm_source=header). For more information on the source of this book, or why it is available for free, please see the project's home page (http://2012books.lardbucket.org/). You can browse or download additional books there. i Chapter 21 Periodic Trends and the s-Block Elements In previous chapters, we used the principles of chemical bonding, thermodynamics, and kinetics to provide a conceptual framework for understanding the chemistry of the elements. Beginning in Chapter 21 "Periodic Trends and the ", we use the periodic table to guide our discussion of the properties and reactions of the elements and the synthesis and uses of some of their commercially important compounds. -

Single Crystal X-Ray Structure Analyses of Thallides: Halide Incorporation and Mixed Alkali Sites in A8tl11x (A = K, Rb, Cs; X = Cl, Br) †
Proceedings Single Crystal X-ray Structure Analyses of Thallides: Halide Incorporation and Mixed Alkali Sites in A8Tl11X (A = K, Rb, Cs; X = Cl, Br) † Stefanie Gärtner 1,2,* and Susanne Tiefenthaler1 1 Institute of Inorganic Chemistry, University of Regensburg, 93040 Regensburg, Germany; [email protected] 2 Central Analytics (X-Ray Dept.), University of Regensburg, 93040 Regensburg, Germany * Correspondence: [email protected]; Tel.: +49-941-943-4446 † Presented at the 1st International Electronic Conference on Crystals, 21–31 May 2018. Available online: https://sciforum.net/conference/IECC_2018. Published: 21 May 2018 Abstract: A8Tl11 (A = alkali metal) compounds have been known since the investigations of Corbett et al. in 1995 and still are matter of current discussions as the compound includes one extra electron referred to the charge of the Tl117− cluster. Attempts to substitute the charge by incorporation of a halide atom succeeded for the lightest homologue of the group, Cs8Ga11Cl, and powder diffraction experiments for the heavier homologues also suggested the formation of analogous compounds. However, X-ray single crystal studies on A8Tl11X to prove this substitution and to provide a deeper insight into the influence on the thallide substructure have not yet been performed, probably due to severe absorption combined with air and moisture sensitivity for this class of compounds. In our contribution we present single crystal X-ray analyses of the new compounds Cs8Tl11Cl0.8, Cs8Tl11Br0.9 and Cs5Rb3Tl11Cl0.5. It is shown that a (partial) incorporation of halide can also be indirectly determined by examination of the Tl-Tl distances for low resolved data sets, e.g., for Cs5.7K2.3Tl11Cl?. -

Substituted Polythiophenes from Highly Reactive Zinc Reagents
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications -- Chemistry Department Published Research - Department of Chemistry 1998 SUBSTITUTED POLYTHIOPHENES FROM HIGHLY REACTIVE ZINC REAGENTS Reuben D. Rieke Follow this and additional works at: https://digitalcommons.unl.edu/chemfacpub Part of the Analytical Chemistry Commons, Medicinal-Pharmaceutical Chemistry Commons, and the Other Chemistry Commons This Article is brought to you for free and open access by the Published Research - Department of Chemistry at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications -- Chemistry Department by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. USO05756653A United States Patent 19 11 Patent Number: 5,756,653 Rieke 45 Date of Patent: May 26, 1998 54 SUBSTITUTED POLYTHIOPHENES FROM J.K. Gawronski, Tetrahedron Letters, 25, 2605 (1984). HGHLY REACTWE ZNC REAGENTS J. Grondin et al., J. Organomet. Chem, 362. 237 (1989). J.J. Habeeb et al., J. Organomet. Chem, 185, 117 (1980). 75) Inventor: Reuben D. Rieke, Lincoln, Nebr. B.H. Han et al., J. Org. Chem, 47, 5030 (1982). B.H. Han et al., J. of the Korean Chemical Society, 29,557 73) Assignee: Board of Regents of the University of (1985). Nebraska, Lincoln, Nebr. S.M. Hannicket al., J. Org. Chem, 48, 3833 (1983). S. W. Hansen et al., J. Fluorine Chem., 35, 415 (1987). 21 Appl. No.: 432,995 P.L. Heinze et al., J. Fluorine Chem., 31. 115 (1986). M. Isobe et al., Chem. Lett, 679 (1977). 22 Filed: May 2, 1995 R.A. Kjonaas et al., J. Org. Chem, 51,3993 (1986). -

ESR of Alkali Metal Dinitrobenzene Salts in Dimethoxyetbane DME*
400 LETTERS TO THE EDITOR J. CHEM. PHYS., VOL. 46, 1967 atoms give 38, of which six must be used for the to 0.18 G at -50°C. The potassium splitting decreases halogens leaving 32 for the metallic cluster. from 0.21 to 0.13 G, and the cesium splitting from 2.46 From the agreement between theory and observa to 2.04 G, over this temperature range. We have ob tion the conclusion is drawn that "electron in a box" served a similar temperature dependence for the alkali theory can be useful in understanding metal atom metal salts of o-dinitrobenzene and nitrobenzene. In clusters, its simplicity being particularly attractive. addition, for each alkali metal, the metal hyperfine splitting is of the same magnitude in the salts of '" This research was supported by the Chemical Directorate of the U.S. Air Force Office of Scientific Research, Grant No. m-dinitrobenzene, o-dinitrobenzene, and nitrobenzene. AF-AFOSR-245-65. On cooling the Cs salt, the hyperfine lines broaden, 1 F. A. Cotton and T. E. Haas, Inorg. Chern. 3,10 (1964). and at - 60°C a small nitrogen splitting of about 0.25 G 2 R. V. Lindsey, Jr., G. W. Parshall, and U. G. Stolberg, Inorg. Chern. 5, 109 (1966). can be resolved. The other nitrogen splitting is now 8.9 G, and even though two inequivalent nitrogens are observed at -60°C, we still observe an equivalent pair ESR of Alkali Metal Dinitrobenzene Salts of protons. in Dimethoxyetbane DME* Cation motion from one nitro group to the other, or cation motion which rotates the nitro groups in and Cm-YUAN LING AND JULIEN GENDELL out of the molecular plane, could account for these Department of Chemistry, University of Michigan facts. -

Reaction of Potassium Fluoride with Organic Halogen Compounds. I
Reaction of Potassium Fluoride with Organic Halogen Compounds. I) Reactions of Potassium Fluoride with Organic Halides, Acids, aad Esters in presence ef Dimethyl Formamide and their Pyrolytic Decaboxylation in presence of Potassium Fluoride By You Sun Kim Atomic Energy Research Institute, Korea 有機 할로겐 化合物과 弗化加里의 反應 (第1報) 有機 할라어드, 酸 및 에스테르와 弗化加里의 디메칠 호쁨아마이드 溶蝶系 反應 및 高混■■脫炭酸-熱分解反應 金 裕 *善 (1963. 6. 19 受理) Abstract Reactions between potassium fluride with organic halogen-containning carboxylic acids in dimethyl formamide solvent gave a decarboxylation reaction for the case of fluoro carboxylic acids of the type of CF3 COOH, C3F7COOH, and C2F5COOH, whereas an additional partial fluorination together with dimeri zation reaction occured for the chlorine containning acids of the type of CH2CICOOH, CH3CHCICOOH, CHCI2COOH and o-Cl-CeHi-COOH. The phenyl halides showed no reactivity, but the halides with two electron attracting substituents on the benzene ring gave mainly dimerization reaction. The esters and alcohols gave an usual fluorination reaction. The same reactions in absence of the solvent at the elevated temperature increase the yield of the dimerized product and gave the cyclized product, fluorenone, in case of o-chlorobenzoic acid. It was found that the fluorination usually precede the decarboxylation reaction by checking the stiochemical sequence of reaction. Catalytic influence of potassium fluoride were discussed and the mechanism of the reaction was considered. 耍 約 「디메望호름아마이드」溶媒系에서 有機含할로겐化合物을 弗化加里와 反應시켜 본 結果 CFsCOOH, CsF’COOH, CzFQOOH 와 같은 含弗素有機酸에서는 脫炭酸反應이 일어나며, 含鹽素有機酸, CH2C1COOH. CH3CHC1COOH, CHC12- COOH 및 o-CK사LCOOH 은 一部 弗化反應이 일어 나고 雙合어imerization) 反應이 隨伴된다는 것을 究明하였다. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Structural Evaluation and Solution Integrity of Alkali Metal Salt Complexes of the Manganese 12-Metallacrown-4 (12-MC-4) Structural Type
6184 Inorg. Chem. 1996, 35, 6184-6193 Structural Evaluation and Solution Integrity of Alkali Metal Salt Complexes of the Manganese 12-Metallacrown-4 (12-MC-4) Structural Type Brian R. Gibney,† Hsin Wang, Jeff W. Kampf, and Vincent L. Pecoraro* Willard H. Dow Laboratories, Department of Chemistry, The University of Michigan, Ann Arbor, Michigan 48109-1055 ReceiVed April 4, 1996X The preparation of a variety of salt complexes of [12-MCMn(III)N(shi)-4] (1) provides the structural basis for the first quantitative investigation of the cation and anion selectivity of metallacrowns. The preparation, X-ray crystal - - structures, and solution integrities of crystalline salts (LiCl2)[12-MCMn(III)N(shi)-4] ([(LiCl2)‚1] ), (Li(trifluoro- + + acetate))[12-MCMn(III)N(shi)-4] ([(LiTFA)‚1]), (Li)[12-MCMn(III)N(shi)-4] ([(Li)‚1] ), (NaBr)2[12-MCMn(III)N(shi)-4] ([(NaBr)2‚1]), and (KBr)2[12-MCMn(III)N(shi)-4] ([(KBr)2‚1]) of the metallacrown [12-MCMn(III)N(shi)-4] (1) are described. Each salt complex of the metallacrown forms from a generic one-step, high-yield synthesis giving 1:1 metal:metallacrown adducts with lithium and 2:1 metal:metallacrown complexes with sodium and potassium ions. On the basis of synthetic preference, the trend for the cation affinity is Li+ > Na+ > K+ and that for anion - - - - - affinity is Cl > Br > TFA > F I3 . The 12-metallacrown-4 structural parameters compare favorably with those of 12-crown-4, an organic crown≈ ether, as well as with those of the topologically similar alkali metal complexes of porphyrin and phthalocyanine dianions, solidifying the structural analogy between metallacrowns and crown ethers. -

Structural Chemistry of Halide Including Thallides A8tl11x1− N
crystals Article Structural Chemistry of Halide including Thallides A8Tl11X1−n (A = K, Rb, Cs; X = Cl, Br; n = 0.1–0.9) Stefanie Gärtner 1,2,* ID , Susanne Tiefenthaler 1, Nikolaus Korber 1 ID , Sabine Stempfhuber 2 and Birgit Hischa 2 1 Institute of Inorganic Chemistry, University of Regensburg, 93040 Regensburg, Germany; [email protected] (S.T.); [email protected] (N.K.) 2 Central Analytics, X-ray Crystallography Dept., University of Regensburg, 93040 Regensburg, Germany; [email protected] (S.S.); [email protected] (B.H.) * Correspondence: [email protected]; Tel.: +49-941-943-4446 Received: 23 July 2018; Accepted: 7 August 2018; Published: 10 August 2018 Abstract: A8Tl11 (A = alkali metal) compounds have been known since the investigations of Corbett et al. in 1995 and are still a matter of current discussions as the compound includes one extra 7− electron referred to the charge of the Tl11 cluster. Attempts to substitute this additional electron by incorporation of a halide atom succeeded in the preparation of single crystals for the lightest triel homologue of the group, Cs8Ga11Cl, and powder diffraction experiments for the heavier homologues also suggested the formation of analogous compounds. However, X-Ray single crystal studies on A8Tl11X to prove this substitution and to provide a deeper insight into the influence on the thallide substructure have not yet been performed, probably due to severe absorption combined with air and moisture sensitivity for this class of compounds. Here, we present single crystal X-Ray structure analyses of the new compounds Cs8Tl11Cl0.8, Cs8Tl11Br0.9, Cs5Rb3Tl11Cl0.5, Cs5.7K2.3Tl11Cl0.6 and K4Rb4Tl11Cl0.1. -

(12) United States Patent (10) Patent No.: US 6,730,210 B2 Thompson Et Al
USOO673021 OB2 (12) United States Patent (10) Patent No.: US 6,730,210 B2 Thompson et al. (45) Date of Patent: May 4, 2004 (54) LOW TEMPERATURE ALKALI METAL GB 3.71946 5/1932 ELECTROLYSIS GB 732906 6/1955 JP 5104.0041 B 4/1976 (75) Inventors: Jeffery S. Thompson, Wilmington, DE E. : A sE. (US); Howard M. Blank, Wilmington, JP 57OO7438 A 1/1982 DE (US); Walter John Simmons, JP 04.0881.88 A 3/1993 Martinsburg, WV (US); Oswald Robert Bergmann, Wilmington, DE (US) OTHER PUBLICATIONS Sodium. Its Manufacture. Properties and Uses, bv. Marshall (73)73) ASSignee : CompE. I. du Any Pont ". d Nine, y,d Sittig, Americans Chemical, PropSociety Monograph ,Series, Dy Rein s s hold Publishing Corp., New York (1956) (Book, not sub (*) Notice: Subject to any disclaimer, the term of this mitted). No month avail. patent is extended or adjusted under 35 Electrochemical Engineering, by C.L. Mantell, U.S.C. 154(b) by 138 days. McGraw-Hill Book Co., Inc., New York, Toronto, London (1960) (Book, not submitted). No month avail. Plating and Stripping of Sodium from a Room Temperature (21) Appl. No.: 10/046,485 1,2-Dimethyl-3-propylimidazolium Chloride Melt, J. Elec (22) Filed: Jan. 14, 2002 trochem. Soc. vol. 143, No. 7, pp. 2262-2266, Jul. 1996. O O Room-temperature Ionic liquids. Solvents for Synthesis and (65) Prior Publication Data catalysis, Thomas Welton, Chemical Reviews, 99, US 2002/0088719 A1 Jul. 11, 2002 2071-2083 (1999). No month avail. Room Temperature Inorganic “Quasi-Molten Salts' as Related U.S.