Product Safety Summary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
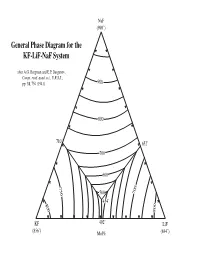
General Phase Diagram for the KF-Lif-Naf System
NaF (990˚) General Phase Diagram for the KF-LiF-NaF System after A.G. Bergman and E.P. Dergunov, Compt. rend. acad. sci., U.R.S.S., pp. 31, 754 (1941). 900 800 710˚ 652˚ 700 600 750 500 700 454˚ 800 800 KF 492˚ LiF (856˚) Mol% (844˚) Sodium Fluoride NaF 950˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 940˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 930˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 920˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 910˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 900˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 890˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 880˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 870˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 860˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 850˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 840˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 830˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 820˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 810˚ 810˚ ˚ KF 810 LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 800˚ 800˚ ˚ KF 800 LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 790˚ 790˚ ˚ KF 790 LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 780˚ 780˚ ˚ LiF KF 780 Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 770˚ 770˚ ˚ LiF KF 770 Potassium Fluoride -

CA004600 SDS.Pdf
Safety Data Sheet SECTION 1: Identification 1.1. Product Identifier Trade Name or Designation: Ammonium Bifluoride, 15.5 g/L, Aqueous Product Number: A-0046 Other Identifying Product Numbers: A-0046-4L 1.2. Recommended Use and Restrictions on Use General Laboratory Reagent 1.3. Details of the Supplier of the Safety Data Sheet Company: Reagents Inc. Address: 4746 Sweden Road Charlotte, NC 28224 USA Telephone: 800-732-8484 1.4. Emergency Telephone Number (24 hr) CHEMTREC (USA) 800-424-9300 CHEMTREC (International) 1+ 703-527-3887 SECTION 2: Hazard(s) Identification 2.1. Classification of the Substance or Mixture (in accordance with OSHA HCS 29 CFR 1910.1200) For the full text of the Hazard and Precautionary Statements listed below, see Section 16. Hazard Hazard Class Category Statement Precautionary Statements Skin Corrosion / Irritation Category 1 H314 P260, P264, P280, P301+P330+P331, P303+P361+P353, P363, P304+P340, P310, P321, P305+P351+P338, P405, P501 Eye Damage / Irritation Category 1 H318 P280, P305+P351+P338, P310 Specific Target Organs/Systemic Toxicity Following Single Category 1 H370 P260, P264, P270, P307+P311, P321, P405, Exposure P501 Specific Target Organs/Systemic Toxicity Following Repeated Category 2 H373 P260, P314, P501 Exposure Corrosive to Metals Category 1 H290 P234, P390, P406 Product Number: A-0046 Page 1 of 10 Safety Data Sheet 2.2. GHS Label Elements Pictograms: Signal Word: Danger Hazard Statements: Hazard Number Hazard Statement H290 May be corrosive to metals. H314 Causes severe skin burns and eye damage. H318 Causes serious eye damage. H370 Causes damage to organs. H373 May cause damage to organs through prolonged or repeated exposure. -

Ammonium Bifluoride CAS No
Product Safety Summary Ammonium Bifluoride CAS No. 1341-49-7 This Product Safety Summary is intended to provide a general overview of the chemical substance. The information on the summary is basic information and is not intended to provide emergency response information, medical information or treatment information. The summary should not be used to provide in-depth safety and health information. In-depth safety and health information can be found in the Safety Data Sheet (SDS) for the chemical substance. Names • Ammonium bifluoride (ABF) • Ammonium difluoride • Ammonium acid fluoride • Ammonium hydrogen difluoride • Ammonium fluoride compound with hydrogen fluoride (1:1) Product Overview Solvay Fluorides, LLC does not sell ammonium bifluoride directly to consumers. Ammonium bifluoride is used in industrial applications and in other processes where workplace exposures can occur. Ammonium bifluoride (ABF) is used for cleaning and etching of metals before they are further processed. It is used as an oil well acidifier and in the etching of glass or cleaning of brick and ceramics. It may also be used for pH adjustment in industrial textile processing or laundries. ABF is available as a solid or liquid solution (in water). Ammonium bifluoride is a corrosive chemical and contact can severely irritate and burn the skin and eyes causing possible permanent eye damage. Breathing ammonium bifluoride can severely irritate and burn the nose, throat, and lungs, causing nosebleeds, cough, wheezing and shortness of breath. On contact with water or moist skin, ABF can release hydrofluoric acid, a very dangerous acid. Inhalation or ingestion of large amounts of ammonium bifluoride can cause nausea, vomiting and loss of appetite. -

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

United States Patent Office Patented July 1, 1969
3,453,337 United States Patent Office Patented July 1, 1969 1. 2 3,453,337 FLUORINATION OF HALOGENATED The presence in the reaction mixture of the two fluo ORGANIC COMPOUNDS rides, or the complex fluoride enables better yields of Royston Henry Bennett and David Walter Cottrell, Ayon highly fluorinated products to be obtained under less mouth, England, assignors to Imperial Smelting Cor severe reaction condions, markedly increases the amount poration (N.S.C.) Limited, London, England, a British of fluorination reagent reacted under otherwise similar company conditions and enables the fluorination reaction to be No brawing. Filed Feb. 19, 1965, Ser. No. 434,128 carried out (for the same yield of product) at a lower Claims priority, application Great Britain, Feb. 26, 1964, temperature with the consequent use of less costly ma 7,932/64 terials and techniques of reactor construction. The pres Int, C. C07c 25/04 10 ence of the fluorides enables the vapor phase reaction U.S. C. 260-650 2 Claims to be carried out (for the same yields) at lower pressure This invention relates to the fluorination of organic than the pressure involved in the reactions using only halogen compounds and more especially to a process the alkali metal fluorides as proposed hitherto. The fur for the production of highly fluorinated aromatic com ther possibility of using a continuous flow apparatus such pounds by the replacement of higher halogen atoms in 15 as a fluidised reactor will be apparent to those familiar halogeno-aromatic compounds by fluorine atoms. with the art. The presence of the two fluorides or com Aromatic halogenocarbons containing carbon and halo plex fluoride enables a lower temperature to be em gen atoms only can be reacted with alkali fluorides ployed than was hitherto believed to be necessary, with under various conditions to give yields of halofluoro a consequent reduction in the extent of thermal degrada aromatic compounds. -

Ammonium Bifluoride ID: C1-102
Material Safety Data Sheet Material Name: Ammonium Bifluoride ID: C1-102 * * * Section 1 - Chemical Product and Company Identification * * * Chemical Name: Ammonium Bifluoride, Technical Flake Grade Product Use: For Commercial Use Synonyms: Ammonium Fluoride; Ammonium Hydrogen Fluoride; Ammonium hydrogendifluoride; Ammonium Difluoride; Acid Ammonium Fluoride. Supplier Information Chem One Ltd. Phone: (713) 896-9966 14140 Westfair East Dr Fax: (713) 896-7540 Houston, Texas 77041-1104 Emergency # (800) 424-9300 or (703) 527-3887 General Comments: FOR COMMERCIAL USE ONLY; NOT TO BE USED AS A PESTICIDE. NOTE: Emergency telephone numbers are to be used only in the event of chemical emergencies involving a spill, leak, fire, exposure, or accident involving chemicals. All non-emergency questions should be directed to customer service. * * * Section 2 - Composition / Information on Ingredients * * * CAS # Component Percent 1341-49-7 Ammonium Bifluoride > 94 12125-01-8 Ammonium Fluoride 4 Component Related Regulatory Information This product may be regulated have exposure limits or other information identified as the following: Fluorides (16984-48- 8), Fluorides, inorganic. Component Information/Information on Non-Hazardous Components This product is considered hazardous under 29 CFR 1910.1200 (Hazard Communication). * * * Section 3 - Hazards Identification * * * Emergency Overview Ammonium Bifluoride is a white, solid that consists of crystals or flakes with a pungent odor. This product is corrosive and causes severe irritation and burning of the eyes, skin and mucous membranes. Harmful or fatal if swallowed, inhaled or if absorbed through the skin. Chronic, low level exposure can lead to bone or dental fluorosis. Fire may produce irritating, corrosive and/or toxic vapors (e.g. ammonia, hydrogen fluoride, and nitrogen oxides). -

Massachusetts Chemical Fact Sheet
Massachusetts Chemical Fact Sheet on the heart and lungs, including pulmonary hemorrhage, Hydrogen Fluoride pulmonary edema, and bronchiolar ulceration. Deaths associated with HF exposure generally result either from This fact sheet is part of a series of chemical fact sheets 2 developed by TURI to help Massachusetts companies, pulmonary edema or from cardiac arrhythmias. community organizations and residents understand a Accidental releases have caused severe respiratory and chemical’s use and health/environmental effects, gastrointestinal symptoms among residents that live near as well as the availability of safer alternatives. the facility.2 Overview TABLE 1: HF Facts Chemical Formula HF Hydrogen fluoride (HF), also known as hydrofluoric acid, CAS Number 7664-39-3 is used primarily for metal cleaning and etching in o o Massachusetts. Nationally, HF is mainly used to Vapor Pressure 760 mm Hg at 68 F (20 C) manufacture chemical refrigerants. Miscible in water; soluble in Solubility ether, soluble in many organic HF is highly corrosive to all tissues and any contact with solvents HF liquid or vapor can cause severe burns (sometimes with Flash point Nonflammable delayed onset), necrosis, and death. Skin contact with HF Reacts violently with strong bases and many other may not cause immediate pain, so systemic poisoning can Reactivity compounds; reacts with water begin before the person is aware of the exposure. and steam to produce toxic and corrosive gases In 2017, Massachusetts facilities subject to TURA reported Colorless, fuming liquid or gas the use of over 230,000 pounds of HF. HF is designated at room temperature with a as a Higher Hazard Substance (HHS) under the Toxics Description sharp, irritating odor that Use Reduction Act (TURA), which lowered the humans can detect at low concentrations (0.04-0.13ppm)4 reporting threshold to 1,000 pounds/year, effective January 2016. -

Acutely / Extremely Hazardous Waste List
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extemely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extemely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extemely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extemely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extemely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extemely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extemely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extemely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extemely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extemely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extemely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extemely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extemely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Reaction of Potassium Fluoride with Organic Halogen Compounds. I
Reaction of Potassium Fluoride with Organic Halogen Compounds. I) Reactions of Potassium Fluoride with Organic Halides, Acids, aad Esters in presence ef Dimethyl Formamide and their Pyrolytic Decaboxylation in presence of Potassium Fluoride By You Sun Kim Atomic Energy Research Institute, Korea 有機 할로겐 化合物과 弗化加里의 反應 (第1報) 有機 할라어드, 酸 및 에스테르와 弗化加里의 디메칠 호쁨아마이드 溶蝶系 反應 및 高混■■脫炭酸-熱分解反應 金 裕 *善 (1963. 6. 19 受理) Abstract Reactions between potassium fluride with organic halogen-containning carboxylic acids in dimethyl formamide solvent gave a decarboxylation reaction for the case of fluoro carboxylic acids of the type of CF3 COOH, C3F7COOH, and C2F5COOH, whereas an additional partial fluorination together with dimeri zation reaction occured for the chlorine containning acids of the type of CH2CICOOH, CH3CHCICOOH, CHCI2COOH and o-Cl-CeHi-COOH. The phenyl halides showed no reactivity, but the halides with two electron attracting substituents on the benzene ring gave mainly dimerization reaction. The esters and alcohols gave an usual fluorination reaction. The same reactions in absence of the solvent at the elevated temperature increase the yield of the dimerized product and gave the cyclized product, fluorenone, in case of o-chlorobenzoic acid. It was found that the fluorination usually precede the decarboxylation reaction by checking the stiochemical sequence of reaction. Catalytic influence of potassium fluoride were discussed and the mechanism of the reaction was considered. 耍 約 「디메望호름아마이드」溶媒系에서 有機含할로겐化合物을 弗化加里와 反應시켜 본 結果 CFsCOOH, CsF’COOH, CzFQOOH 와 같은 含弗素有機酸에서는 脫炭酸反應이 일어나며, 含鹽素有機酸, CH2C1COOH. CH3CHC1COOH, CHC12- COOH 및 o-CK사LCOOH 은 一部 弗化反應이 일어 나고 雙合어imerization) 反應이 隨伴된다는 것을 究明하였다. -

Ammonium Bifluoride
SAFETY DATA SHEET AMMONIUM BIFLUORIDE Revision Date 04/20/2015 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier - Trade name AMMONIUM BIFLUORIDE - Chemical Name Ammonium hydrogendifluoride Distributed By: - Synonyms Ammonium hydrogen fluoride - Molecular formula NH4F.HF SAL Chemical 1.2 Relevant identified uses of the substance or mixture and uses advised against 3036 Birch Drive Uses of the Substance / Mixture Weirton, WV 26062 - Cleaning agent 304-748-8200 - Metal treatment - Non-metal-surface treatment products - Oil & gas industry - Chemical intermediate 1.3 Details of the supplier of the safety data sheet Company SOLVAY FLUORIDES, LLC 3333 RICHMOND AVENUE 77098-3099, HOUSTON USA Tel: +1-800-7658292; +1-713-5256700 Fax: +1-713-5257805 Prepared by Solvay Product Stewardship (see Telephone number above) Date Prepared 04/20/2015 1.4 Emergency telephone FOR EMERGENCIES INVOLVING A SPILL, LEAK, FIRE, EXPOSURE OR ACCIDENT CONTACT: CHEMTREC 800-424- 9300 within the United States and Canada, or 703-527-3887 for international collect calls. SECTION 2: Hazards identification 2.1 Emergency overview Appearance Form : flakes, strongly hygroscopic Physical state: solid Color: white white Odor: pungent Warning statements - Toxic if swallowed. - Causes burns. - Hazardous decomposition products formed under fire conditions. - Hydrogen fluoride - Chronic exposure may entail dental or skeletal fluorosis 2.2 Potential Health Effects P00000020030 Version : 1.03 / CA ( Z8 ) 1 / 16 SAFETY DATA SHEET AMMONIUM BIFLUORIDE Revision Date 04/20/2015 Inhalation effect - Inhalation of vapors is irritating to the respiratory system, may cause throat pain and cough. - Breathing difficulties - Aspiration may cause pulmonary edema and pneumonitis. -

Potassium Hydrogen Difluoride Product Information Cas № 7789-29
POTASSIUM HYDROGEN DIFLUORIDE PRODUCT INFORMATION CAS № 7789-29 POTASSIUM HYDROGEN DIFLUORIDE A TOXIC SUBSTANCE. Fire- and explosion-safe. Hygroscopic. Please refer to manufacturer’s SDS for further information POTASSIUM HYDROGEN DIFLUORIDE CAS № 7789-29-9 APPLICATIONS: PACKAGING: NUCLEAR INDUSTRY Polyethylene bags AIRCRAFT ENGINES inserted in convolute drums. FLUORINE CHEMISTRY Net weight 35 Kg. FERROUS AND NON- FERROUS METALLURGY Guaranteed shelf-life GLASS INDUSTRY 3 years following the date of manufacture provided that the storage Technology: reaction of an excess of concentrated hydrofluoric acid with conditions are met. concemtrated solution of potassium hydroxide KOH + 2 HF 2 KHF2 + CO2 ELEMENT Standard COMPONENT Standard as per (GOST 10067-80) TU 95-183-90 LU Mass fraction of potassium bifluoride (KF•HF), % 98-102 Mass fraction of hydrogen fluoride, %, within 25.0-26.0 the range of Mass fraction of clorides (Cl), %, max. 0,005 Mass fraction of silicon (Si), %, max. 0.1 Mass fraction of sulfates (SO4), %, max. 0,01 Mass fraction of sulfates (SO4), %, max. 0.1 Mass fraction of iron (Fe), %, max. 0,001 Mass fraction of iron (Fe), %, max. 0.10 Mass fraction of silicon (Si), %, max. 0,01 Mass fraction of insoluble sediment, %, max. 0.5 Mass fraction of lead, copper and manganese 0,001 (Pb+Cu+Mn), %, max. Appearance: white or grey powder. Lumps of various forms and sizes in the powder are acceptable. Manufactured in accordance with GOST 10067-80 “Potassium Hydrogen Difluoride” or TU 95-183-90 LU Production capacity: up to 180 tpa AECC is a ROSATOM Fuel Company «TVEL» Enterprise The plant is deemed to be one of the most state-of-the-art hi-tech companies of Russia’s nuclear industry with 60-year experience in manufacturing hi-quality products, and it is continuously expanding its presence on the domestic and international markets. -

XCEL NF Flux, 09-NF-00
JOHNSON MANUFACTURING COMPANY Safety Data Sheet To comply with 29CFR 1910.1200 OSHA's Hazard Communication Standard XCEL NF Flux, 09-NF-00 1. PRODUCT AND COMPANY INFORMATION Johnson Manufacturing Company Emergency Telephone 1-(563)-289-5123 114 Lost Grove Road CHEMTREC AFTER HOURS 1-(800)-424-9300 Princeton IA 52768 Revised 1/1/2021 by JMC Product Safety 2. HAZARD IDENTIFICATION GHS Classification: Acute Tox. 3 Chronic tox 3 Skin corr 3 Eye corr 3 GHS Label Elements: POTASSIUM BIFLUORIDE & BORIC ACID DANGER H Codes: H301, H314, H331, H360 Toxic if swallowed Toxic if inhaled Causes severe skin burns & eye damage May damage fertility or the unborn child P Codes: P264, 270, 201, 202, 281, 308+313, 280, 301+312, 330, 501, 271, 311, $03+233, 302, 352, 308+313, 363, 304+340, 321, 405, Do not breath dust/mist/vapors/fumes/spray. Do not get in eyes, on skin, or on clothing. Use in a well ventilated area. Wear protective gloves/protective clothing/eye protection/face protection. In case of inadequate ventilation use respiratory protection. Do not eat, drink or smoke when using this product. IF SWALLOWED: Rinse mouth. DO NOT induce vomiting. Immediately call a POISON CENTER/Doctor. IF ON SKIN (or hair): Take off immediately all contaminated clothing. Wash with soap & water. If skin irritation occurs, get medical advise/attention. IF INHALED: Remove victim to to fresh air and keep comfortable for breathing. Immediately call a POISON CENTER/Doctor. IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do.