A,G. 22, 1950 J.H. SIMONS 2,519,983 1010.0001
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
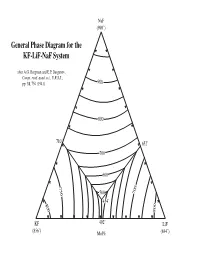
General Phase Diagram for the KF-Lif-Naf System
NaF (990˚) General Phase Diagram for the KF-LiF-NaF System after A.G. Bergman and E.P. Dergunov, Compt. rend. acad. sci., U.R.S.S., pp. 31, 754 (1941). 900 800 710˚ 652˚ 700 600 750 500 700 454˚ 800 800 KF 492˚ LiF (856˚) Mol% (844˚) Sodium Fluoride NaF 950˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 940˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 930˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 920˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 910˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 900˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 890˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 880˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 870˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 860˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 850˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 840˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 830˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 820˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 810˚ 810˚ ˚ KF 810 LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 800˚ 800˚ ˚ KF 800 LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 790˚ 790˚ ˚ KF 790 LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 780˚ 780˚ ˚ LiF KF 780 Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 770˚ 770˚ ˚ LiF KF 770 Potassium Fluoride -

Use of Solvents for Pahs Extraction and Enhancement of the Pahs Bioremediation in Coal- Tar-Contaminated Soils Pak-Hing Lee Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 2000 Use of solvents for PAHs extraction and enhancement of the PAHs bioremediation in coal- tar-contaminated soils Pak-Hing Lee Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Environmental Engineering Commons Recommended Citation Lee, Pak-Hing, "Use of solvents for PAHs extraction and enhancement of the PAHs bioremediation in coal-tar-contaminated soils " (2000). Retrospective Theses and Dissertations. 13912. https://lib.dr.iastate.edu/rtd/13912 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter fece, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quaiity of the copy submitted. Broken or indistinct print colored or poor quality illustrations and photographs, print bleedthrough, substeindard margins, and improper alignment can adversely affect reproduction. In the unlilcely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. -

Supporting Information of Polycyclic Aromatic Hydrocarbons (Pahs) In
Supporting Information of Polycyclic aromatic hydrocarbons (PAHs) in aerosols over the central Himalayas along two south-north transects Peng Fei Chen1,5, Chao Liu Li1, Shi Chang Kang2,3*, Maheswar Rupakheti4, Arnico K Panday6, Fang Ping Yan2,5, Quan Lian Li2, Qiang Gong Zhang1,3, Jun Ming Guo1,5, Dipesh Rupakheti1,5, Wei Luo7 1Key Laboratory of Tibetan Environment Changes and Land Surface Processes, Institute of Tibetan Plateau Research, Chinese Academy of Sciences, Beijing 100101, China 2State Key Laboratory of Cryospheric Science, Cold and Arid Regions Environmental and Engineering Research Institute, Lanzhou 730000, China 3Center for Excellence in Tibetan Plateau Earth Sciences, Chinese Academy of Sciences, Beijing 100085, China; 4Institute for Advanced Sustainability Studies, Potsdam 14467, Germany 5University of Chinese Academy of Sciences, Beijing 100039, China 6International Centre for Integrated Mountain Development, Kathmandu, Nepal 7State Key Lab of Urban and Regional Ecology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China Correspondence to: S. Kang ([email protected]) Text SI-1 Sample extraction and analysis Text SI-2 Quality control Table SI-1 Percentage contribution (%) of each species to total PAHs in the atmosphere over the Himalayas. Text SI-1 Sample extraction and analysis A quarter of each filter was cut into pieces, placed into a glass tube, and immersed in 20 mL of dichloromethane (DCM) and n-hexane (1:1). The extraction was performed by sonication twice for 30 min at 27 °C. Every single sample was spiked with deuterated PAHs (naphthalene-d8, acenaphthene-d10, phenanthrene-d10, chrysene-d12, and perylene-d12) as recovery surrogates. -

Material Safety Data Sheet Prepared to US OSHA, CMA, ANSI, Canadian WHMIS and EU Standards
MSDS NUMBER: 1201 Fluorine Excimer Laser Mix (1.0% or less Fluorine) Material Safety Data Sheet Prepared to US OSHA, CMA, ANSI, Canadian WHMIS and EU Standards SECTION 1. PRODUCT IDENTIFICATION PRODUCT NAME: Fluorine Excimer Laser Mix (1.0% or less Fluorine) US DOT NAME: Compressed Gas, n.o.s. (Fluorine, other gas) UN 1956 (see Sec. 14) CHEMICAL NAME: Mixture of Fluorine (1.0% or Less) and Argon, Krypton or Xenon balance Helium, Neon or Nitrogen FORMULA: Argon = Ar; Fluorine = F2; Helium = He; Krypton = Kr; Neon = Ne; Nitrogen = N2; SYNONYMS: Not Applicable US MANUFACTURER: Linde LLC 575 Mountain Ave. Murray Hill, NJ 07974 Phone: 908-464-8100 lindeus.com 24 HOUR EMERGENCY CONTACT, CHEMTREC: 800/424-9300, 703/527-3887 For additional product information contact your customer service representative. PRODUCT USE: In Excimer Laser and Research and Development ALL WHMIS required information is included in appropriate sections based on the ANSI Z400.1-2004 format. This product has been classified in accordance with the hazard criteria of the CPR and the MSDS contains all the information required by the CPR. The product is also classified per all applicable EU Directives through EC 1907: 2006 SECTION 2. HAZARD IDENTIFICATION EU LABELING AND CLASSIFICATION: This gas mixture meets the definition of hazardous according to the criteria of the European Union Council Directive 67/548/EEC and based on current guidelines under EC 1907: 2006. EU CLASSIFICATION: Xn (Harmful) EU RISK PHRASES: R: 20; R: 21; R: 36/37/38 EU SAFETY PHRASES: S: (1/2)*; S: 7/9, S: 26, S: 36/37/39, S: 45 See Section 15 for full definition of Risk and Safety Phrases. -

Effect of Epoxide Content on the Vulcanizate Structure of Silica-Filled Epoxidized Natural Rubber (ENR) Compounds
polymers Article Effect of Epoxide Content on the Vulcanizate Structure of Silica-Filled Epoxidized Natural Rubber (ENR) Compounds Gyeongchan Ryu 1, Donghyuk Kim 1, Sanghoon Song 1, Kiwon Hwang 1, Byungkyu Ahn 2 and Wonho Kim 1,* 1 School of Chemical Engineering, Pusan National University, Busan 46241, Korea; [email protected] (G.R.); [email protected] (D.K.); [email protected] (S.S.); [email protected] (K.H.) 2 Hankook Tire & Technology Co., Ltd., R&D Center, 50 Yuseong-daero 935 beon-gil, Yuseong-gu, Daejeon 34127, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-51-510-3190 Abstract: The demand for truck–bus radial (TBR) tires with enhanced fuel efficiency has grown in recent years. Many studies have investigated silica-filled natural rubber (NR) compounds to address these needs. However, silica-filled compounds offer inferior abrasion resistance compared to carbon black-filled compounds. Further, the use of NR as a base rubber can hinder silanization and coupling reactions due to interference by proteins and lipids. Improved silica dispersion be achieved without the use of a silane coupling agent by introducing epoxide groups to NR, which serve as silica-affinitive functional groups. Furthermore, the coupling reaction can be promoted by facilitating chemical interaction between the hydroxyl group of silica and the added epoxide groups. Thus, this study evaluated the properties of commercialized NR, ENR-25, and ENR-50 compounds with or without an added silane coupling agent, and the filler–rubber interaction was quantitatively calculated using vulcanizate structure analysis. The increased epoxide content, when the silane coupling agent was not used, improved silica dispersion, abrasion resistance, fuel efficiency, and wet Citation: Ryu, G.; Kim, D.; Song, S.; grip. -

Organic Chemistry Name Formula Isomers Methane CH 1 Ethane C H
Organic Chemistry Organic chemistry is the chemistry of carbon. The simplest carbon molecules are compounds of just carbon and hydrogen, hydrocarbons. We name the compounds based on the length of the longest carbon chain. We then add prefixes and suffixes to describe the types of bonds and any add-ons the molecule has. When the molecule has just single bonds we use the -ane suffix. Name Formula Isomers Methane CH4 1 Ethane C2H6 1 Propane C3H8 1 Butane C4H10 2 Pentane C5H12 3 Hexane C6H14 5 Heptane C7H16 9 Octane C8H18 18 Nonane C9H20 35 Decane C10H22 75 Isomers are compounds that have the same formula but different bonding. isobutane n-butane 1 Naming Alkanes Hydrocarbons are always named based on the longest carbon chain. When an alkane is a substituent group they are named using the -yl ending instead of the -ane ending. So, -CH3 would be a methyl group. The substituent groups are named by numbering the carbons on the longest chain so that the first branching gets the lowest number possible. The substituents are listed alphabetically with out regard to the number prefixes that might be used. 3-methylhexane 1 2 3 4 5 6 6 5 4 3 2 1 Alkenes and Alkynes When a hydrocarbon has a double bond we replace the -ane ending with -ene. When the hydrocarbon has more than three carbon the position of the double bond must be specified with a number. 1-butene 2-butene Hydrocarbons with triple bonds are named basically the same, we replace the -ane ending with -yne. -

United States Patent Office Patented July 1, 1969
3,453,337 United States Patent Office Patented July 1, 1969 1. 2 3,453,337 FLUORINATION OF HALOGENATED The presence in the reaction mixture of the two fluo ORGANIC COMPOUNDS rides, or the complex fluoride enables better yields of Royston Henry Bennett and David Walter Cottrell, Ayon highly fluorinated products to be obtained under less mouth, England, assignors to Imperial Smelting Cor severe reaction condions, markedly increases the amount poration (N.S.C.) Limited, London, England, a British of fluorination reagent reacted under otherwise similar company conditions and enables the fluorination reaction to be No brawing. Filed Feb. 19, 1965, Ser. No. 434,128 carried out (for the same yield of product) at a lower Claims priority, application Great Britain, Feb. 26, 1964, temperature with the consequent use of less costly ma 7,932/64 terials and techniques of reactor construction. The pres Int, C. C07c 25/04 10 ence of the fluorides enables the vapor phase reaction U.S. C. 260-650 2 Claims to be carried out (for the same yields) at lower pressure This invention relates to the fluorination of organic than the pressure involved in the reactions using only halogen compounds and more especially to a process the alkali metal fluorides as proposed hitherto. The fur for the production of highly fluorinated aromatic com ther possibility of using a continuous flow apparatus such pounds by the replacement of higher halogen atoms in 15 as a fluidised reactor will be apparent to those familiar halogeno-aromatic compounds by fluorine atoms. with the art. The presence of the two fluorides or com Aromatic halogenocarbons containing carbon and halo plex fluoride enables a lower temperature to be em gen atoms only can be reacted with alkali fluorides ployed than was hitherto believed to be necessary, with under various conditions to give yields of halofluoro a consequent reduction in the extent of thermal degrada aromatic compounds. -

19770005666.Pdf
General Disclaimer One or more of the Following Statements may affect this Document This document has been reproduced from the best copy furnished by the organizational source. It is being released in the interest of making available as much information as possible. This document may contain data, which exceeds the sheet parameters. It was furnished in this condition by the organizational source and is the best copy available. This document may contain tone-on-tone or color graphs, charts and/or pictures, which have been reproduced in black and white. This document is paginated as submitted by the original source. Portions of this document are not fully legible due to the historical nature of some of the material. However, it is the best reproduction available from the original submission. Produced by the NASA Center for Aerospace Information (CASI) V NASA TECHNICAL NASA TM X-73983 MEMORANDUM 00 X I-- A SURVEY OF KINETIC DATA OF COMPOUNDS CONTAINING FLUORINE Dana A. Brewer Langley Research Center (NASA-TM-X-73983) A SURVEY OF KINETIC DATA N77-12609 OF COMPOUNDS CONTAINING FLOURINE (NASA) 119 p, HC A06/MF A01 CSCL 04A Unclas G3/46 55838 November 1976 This informal documentation medium is used to provide accelerated or special release of technical information to selected users. The contents may not meet NASA formal editing and publication standards, may be re- vised, or may I be incorporated in another publication. 4WAil NATIONAL AERONAUTICS AND SPACE ADMINISTRATION DEC 1976 LANGLEY RESEARCH CENTER, HAMPTON, VIRGINIA 23665 WEIVED 0M tosp, Sn PACILITY INpUT BRANCH < C' ' r ^| ' 1. Report No. -

Stability Studies of Selected Polycyclic Aromatic Hydrocarbons in Different Organic Solvents and Identification of Their Transformation Products
Polish J. of Environ. Stud. Vol. 17, No. 1 (2008), 17-24 Original Research Stability Studies of Selected Polycyclic Aromatic Hydrocarbons in Different Organic Solvents and Identification of Their Transformation Products D. Dąbrowska1, A. Kot-Wasik2, J. Namieśnik*2 1Polish geological institute, Central Chemical laboratory, warsaw, Poland 2Department of Analytical Chemistry, gdansk university of technology, ul. Narutowicza 11/12, 80-892 gdańsk, Poland Received: May 7, 2007 Accepted: October 1, 2007 Abstract one of the problems in an hPlC laboratory is the preservation of samples and extracts prior to analysis without any changes. It has been found that degradation processes cannot be eliminated entirely, but they can be slowed down considerably. Photodegradation kinetics of fluorene, anthracene and benzo(a)pyrene were studied in various organic solvents. Solvents known as good media to store PAhs for a long time were selected with respect to avoid degradation. in the case of fluorene, 9-fluorenone and 9-hydroxyfluorene were detected as main photoproducts. Formation of anthraquinone and 1,8-dihydroxyanthraquinone during the degradation of anthracene was observed. Benzo(a)pyrene-4,5-dihydrodiol and one of the isomers of hydroxy-BaP-dione as products of benzo(a)pyrene photodegradation have been identified. Keywords: polycyclic aromatic hydrocarbons, photodegradation, degradation products, sample stability Introduction 4 or more rings and their metabolites, have a variety of mutagenic and carcinogenic effects on microorganisms, Polycyclic aromatic hydrocarbons (PAhs) are ubiq- plants and animals, and are classified as compounds with uitous contaminants originating from natural and anthro- significant human health risks [6]. pogenic pyrolysis of organic matter such as forest fires, In the environment, primary removal processes of low automobile exhaust, coal and oil refining processes.t heir molecular weight PAhs are microbial degradation and abundance and persistence in several polluted environ- evaporation. -

INVESTIGATION of POLYCYCLIC AROMATIC HYDROCARBONS (Pahs) on DRY FLUE GAS DESULFURIZATION (FGD) BY-PRODUCTS
INVESTIGATION OF POLYCYCLIC AROMATIC HYDROCARBONS (PAHs) ON DRY FLUE GAS DESULFURIZATION (FGD) BY-PRODUCTS DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Ping Sun, M.S. ***** The Ohio State University 2004 Dissertation Committee: Approved by Professor Linda Weavers, Adviser Professor Harold Walker Professor Patrick Hatcher Adviser Professor Yu-Ping Chin Civil Engineering Graduate Program ABSTRACT The primary goal of this research was to examine polycyclic aromatic hydrocarbons (PAHs) on dry FGD by-products to determine environmentally safe reuse options of this material. Due to the lack of information on the analytical procedures for measuring PAHs on FGD by-products, our initial work focused on analytical method development. Comparison of the traditional Soxhlet extraction, automatic Soxhlet extraction, and ultrasonic extraction was conducted to optimize the extraction of PAHs from lime spray dryer (LSD) ash (a common dry FGD by-product). Due to the short extraction time, ultrasonic extraction was further optimized by testing different organic solvents. Ultrasonic extraction with toluene as the solvent turned out to be a fast and efficient method to extract PAHs from LSD ash. The possible reactions of PAHs under standard ultrasonic extraction conditions were then studied to address concern over the possible degradation of PAHs by ultrasound. By sonicating model PAHs including naphthalene, phenanthrene and pyrene in organic solutions, extraction parameters including solvent type, solute concentration, and sonication time on reactions of PAHs were examined. A hexane: acetone (1:1 V/V) ii mixture resulted in less PAH degradation than a dichloromethane (DCM): acetone (1:1 V/V) mixture. -

Organic Nomenclature: Naming Organic Molecules
Organic Nomenclature: Naming Organic Molecules Mild Vegetable Alkali Aerated Alkali What’s in a name? Tartarin Glauber's Alkahest Alkahest of Van Helmot Fixed Vegetable Alkali Russian Pot Ash Cendres Gravellees Alkali Mild Vegetable Oil of Tartar Pearl Ash Tartar Alkahest of Reapour K2CO3 Alkali of Reguline Caustic Sal Juniperi Potassium Carbonate Ash ood Alkali of Wine Lees Salt of Tachenius W Fixed Sal Tartari Sal Gentianae Alkali Salt German Ash Salt of Wormwood Cineres Clavellati Sal Guaiaci exSal Ligno Alkanus Vegetablis IUPAC Rules for naming organic molecules International Union of Pure and Applied Chemists 3 Name Molecular Prefix Formula Methane CH4 Meth Ethane C2H6 Eth Alkanes Propane C3H8 Prop Butane C4H10 But CnH2n+2 Pentane C5H12 Pent Hexane C6H14 Hex Heptane C7H16 Hept Octane C8H18 Oct Nonane C9H20 Non Decane C10H22 Dec 4 Structure of linear alkanes propane butane pentane hexane heptane octane nonane decane 5 Constitutional Isomers: molecules with same molecular formula but differ in the way in which the atoms are connected to each other 6 Physical properties of constiutional isomers 7 Isomers n # of isomers 1 1 2 1 The more carbons in a 3 1 molecule, the more 4 2 possible ways to put 5 3 them together. 6 5 7 9 8 18 9 35 10 75 15 4,347 25 36,797,588 8 Naming more complex molecules hexane C6H14 9 Naming more complex molecules Step 1: identify the longest continuous linear chain: this will be the root name: this is the root name 2 4 6 longest chain = 6 (hexane) 1 3 5 2 4 3 4 3 2 2 1 5 4 1 3 5 correct 1 incorrect longest chain = 5 (pentane) longest chain = 5 (pentane) 1 3 1 2 2 3 4 4 longest chain = 4 (butane) longest chain = 4 (butane) 10 Naming more complex molecules Step 2: identify all functional group (the groups not part of the “main chain”) CH3 2 4 4 3 2 1 5 1 3 5 CH3 main chain: pentane main chain: pentane CH3 1 3 1 2 2 3 4 4 CH3 CH3 CH3 main chain: butane main chain: butane 11 Alkyl groups: fragments of alkanes H H empty space (point where it H C H H C attaches to something else) H H methane methyl CH4 CH3 12 More generally.. -

Reaction of Potassium Fluoride with Organic Halogen Compounds. I
Reaction of Potassium Fluoride with Organic Halogen Compounds. I) Reactions of Potassium Fluoride with Organic Halides, Acids, aad Esters in presence ef Dimethyl Formamide and their Pyrolytic Decaboxylation in presence of Potassium Fluoride By You Sun Kim Atomic Energy Research Institute, Korea 有機 할로겐 化合物과 弗化加里의 反應 (第1報) 有機 할라어드, 酸 및 에스테르와 弗化加里의 디메칠 호쁨아마이드 溶蝶系 反應 및 高混■■脫炭酸-熱分解反應 金 裕 *善 (1963. 6. 19 受理) Abstract Reactions between potassium fluride with organic halogen-containning carboxylic acids in dimethyl formamide solvent gave a decarboxylation reaction for the case of fluoro carboxylic acids of the type of CF3 COOH, C3F7COOH, and C2F5COOH, whereas an additional partial fluorination together with dimeri zation reaction occured for the chlorine containning acids of the type of CH2CICOOH, CH3CHCICOOH, CHCI2COOH and o-Cl-CeHi-COOH. The phenyl halides showed no reactivity, but the halides with two electron attracting substituents on the benzene ring gave mainly dimerization reaction. The esters and alcohols gave an usual fluorination reaction. The same reactions in absence of the solvent at the elevated temperature increase the yield of the dimerized product and gave the cyclized product, fluorenone, in case of o-chlorobenzoic acid. It was found that the fluorination usually precede the decarboxylation reaction by checking the stiochemical sequence of reaction. Catalytic influence of potassium fluoride were discussed and the mechanism of the reaction was considered. 耍 約 「디메望호름아마이드」溶媒系에서 有機含할로겐化合物을 弗化加里와 反應시켜 본 結果 CFsCOOH, CsF’COOH, CzFQOOH 와 같은 含弗素有機酸에서는 脫炭酸反應이 일어나며, 含鹽素有機酸, CH2C1COOH. CH3CHC1COOH, CHC12- COOH 및 o-CK사LCOOH 은 一部 弗化反應이 일어 나고 雙合어imerization) 反應이 隨伴된다는 것을 究明하였다.