Inorganic Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Material Safety Data Sheet Prepared to US OSHA, CMA, ANSI, Canadian WHMIS and EU Standards
MSDS NUMBER: 1201 Fluorine Excimer Laser Mix (1.0% or less Fluorine) Material Safety Data Sheet Prepared to US OSHA, CMA, ANSI, Canadian WHMIS and EU Standards SECTION 1. PRODUCT IDENTIFICATION PRODUCT NAME: Fluorine Excimer Laser Mix (1.0% or less Fluorine) US DOT NAME: Compressed Gas, n.o.s. (Fluorine, other gas) UN 1956 (see Sec. 14) CHEMICAL NAME: Mixture of Fluorine (1.0% or Less) and Argon, Krypton or Xenon balance Helium, Neon or Nitrogen FORMULA: Argon = Ar; Fluorine = F2; Helium = He; Krypton = Kr; Neon = Ne; Nitrogen = N2; SYNONYMS: Not Applicable US MANUFACTURER: Linde LLC 575 Mountain Ave. Murray Hill, NJ 07974 Phone: 908-464-8100 lindeus.com 24 HOUR EMERGENCY CONTACT, CHEMTREC: 800/424-9300, 703/527-3887 For additional product information contact your customer service representative. PRODUCT USE: In Excimer Laser and Research and Development ALL WHMIS required information is included in appropriate sections based on the ANSI Z400.1-2004 format. This product has been classified in accordance with the hazard criteria of the CPR and the MSDS contains all the information required by the CPR. The product is also classified per all applicable EU Directives through EC 1907: 2006 SECTION 2. HAZARD IDENTIFICATION EU LABELING AND CLASSIFICATION: This gas mixture meets the definition of hazardous according to the criteria of the European Union Council Directive 67/548/EEC and based on current guidelines under EC 1907: 2006. EU CLASSIFICATION: Xn (Harmful) EU RISK PHRASES: R: 20; R: 21; R: 36/37/38 EU SAFETY PHRASES: S: (1/2)*; S: 7/9, S: 26, S: 36/37/39, S: 45 See Section 15 for full definition of Risk and Safety Phrases. -

Short Description of the Gulaim Seisenbaeva´S Research Profile
Short description of the Gulaim Seisenbaeva´s research profile My research work has always been concentrated on development of chemical synthetic approaches to new materials, aiming to influence purpose characteristics of the material by varying chemical and physicochemical parameters of its synthesis. In my projects I am always trying to trace and understand the transformation from molecular precursors to materials with desired properties. The focus of my work is in the search for Molecular Chemistry approaches to nano-level defined materials. During the work at SLU for the last 18 years my research became strongly directed toward the use of bio-based materials and to applications in the Green Sector in the domains of environmental science and protection of the environment, bio-energy and bio-catalysis. My original scientific training was in the field of chemical technology and heterogeneous catalysis. In my Master Thesis entitled “Applied ruthenium and copper-ruthenium catalysts in the liquid phase hydrogenation reactions” I investigated thermal decomposition of metal complexes at different temperatures and the influence of decomposition temperature on the activity of prepared catalysts [1]. Since then I have become interested in molecular chemistry tools for production of functional solid materials, in particular, thermal decomposition mechanisms and their influence on morphology and chemical composition of the resulting products. The Ph.D. research work, “Tricarboxylatouranylates(VI) of protonated amines”, was devoted to the development of synthetic approaches to anionic carboxylate complexes of uranium(VI), and investigation of their thermal decomposition mechanisms leading to formation of highly disperse uranium dioxide for application in nuclear fuel blocks. There have been developed general approaches to the synthesis of anionic carboxylate complexes of uranium(VI) using interaction of uranyl carboxylates with hydrated amines in aqueous solutions of carboxylic acids. -

19770005666.Pdf
General Disclaimer One or more of the Following Statements may affect this Document This document has been reproduced from the best copy furnished by the organizational source. It is being released in the interest of making available as much information as possible. This document may contain data, which exceeds the sheet parameters. It was furnished in this condition by the organizational source and is the best copy available. This document may contain tone-on-tone or color graphs, charts and/or pictures, which have been reproduced in black and white. This document is paginated as submitted by the original source. Portions of this document are not fully legible due to the historical nature of some of the material. However, it is the best reproduction available from the original submission. Produced by the NASA Center for Aerospace Information (CASI) V NASA TECHNICAL NASA TM X-73983 MEMORANDUM 00 X I-- A SURVEY OF KINETIC DATA OF COMPOUNDS CONTAINING FLUORINE Dana A. Brewer Langley Research Center (NASA-TM-X-73983) A SURVEY OF KINETIC DATA N77-12609 OF COMPOUNDS CONTAINING FLOURINE (NASA) 119 p, HC A06/MF A01 CSCL 04A Unclas G3/46 55838 November 1976 This informal documentation medium is used to provide accelerated or special release of technical information to selected users. The contents may not meet NASA formal editing and publication standards, may be re- vised, or may I be incorporated in another publication. 4WAil NATIONAL AERONAUTICS AND SPACE ADMINISTRATION DEC 1976 LANGLEY RESEARCH CENTER, HAMPTON, VIRGINIA 23665 WEIVED 0M tosp, Sn PACILITY INpUT BRANCH < C' ' r ^| ' 1. Report No. -

29, 1946. LH Dawsl -:Y Erm. 2393391
Jann.` 29, 1946. L. H. DAwsl_-:Y_ Erm. 2,393,391 ‘ PRODUCTION oF METAL PERoxIDEs Filed June 8, 1943 INVENTORS NEYS Patented Jan. 29,1946 2,393,891 UNITED .STATES PATENT olf-FICE 2,393,891 PRODUCTION OF METAL PEROXIDES Lynn H. Dawsey, Kenmore, and Hans A. Rudolph, Buiîalo, N. Y., assignors to Bull'alo Electro Chexnical Company, Inc., Tonawanda, N. Y. Application June 8, 1943, Serial No. 490,040 3 Claims. (Cl. 23187) Thisl invention relates to the stabilization of ing a very real combustion hazard. Another dis alkaline earth metal peroxides stabilized against advantage resides in using stabilizers containing deterioration due to absorptionof atmospheric magnesium, aluminum, or zinc, as large propor water andfcarbon- dioxide. It is especially appli tions of foreign metals are lintroduced into the cable in the manufacture of calcium and stron-Y product, which are, of course, undesirable when tium peroxides‘. _ ' i ' the product is to be utilized in special chemical It' is alwell-known fact that the anhydrous syntheses involving polymerization reactions and alkaline earth metal peroxides are hygroscopic, the like. A further disadvantage of the products and when exposed to I.the atmosphere rapidly take of the prior art is their tendency to cake or ball up moisture and/ carbon dioxide with the forma .up when manipulated rather .than remain free tion of crystalline peroxide‘hydrates, hydroxides iiowing. Y y ' and carbonates. The absorption of water and It is an object of the present invention to over l carbon dioxide by the alkaline earth metal per come the previous disadvantages of the prior art. -

Experimental Studies on the Dynamic Memcapacitance Modulation of the Reo3@Res2 Composite Material-Based Diode
nanomaterials Article Experimental Studies on the Dynamic Memcapacitance Modulation of the ReO3@ReS2 Composite Material-Based Diode Joanna Borowiec 1,2,* , Mengren Liu 3 , Weizheng Liang 4, Theo Kreouzis 2 , Adrian J. Bevan 2 , Yi He 1, Yao Ma 1 and William P. Gillin 1,2 1 College of Physics, Sichuan University, Chengdu 610064, China; [email protected] (Y.H.); [email protected] (Y.M.); [email protected] (W.P.G.) 2 Materials Research Institute and School of Physics and Astronomy, Queen Mary University of London, Mile End Road, London E1 4NS, UK; [email protected] (T.K.); [email protected] (A.J.B.) 3 Sichuan University—Pittsburgh Institute, Chengdu 610207, China; [email protected] 4 The Peac Institute of Multiscale Sciences, Chengdu 610031, China; [email protected] * Correspondence: [email protected]; Tel.: +86-028-854-12323 Received: 4 October 2020; Accepted: 19 October 2020; Published: 23 October 2020 Abstract: In this study, both memcapacitive and memristive characteristics in the composite material based on the rhenium disulfide (ReS2) rich in rhenium (VI) oxide (ReO3) surface overlayer (ReO3@ReS2) and in the indium tin oxide (ITO)/ReO3@ReS2/aluminum (Al) device configuration is presented. Comprehensive experimental analysis of the ReO3@ReS2 material properties’ dependence on the memcapacitor electrical characteristics was carried out by standard as well as frequency-dependent current–voltage, capacitance–voltage, and conductance–voltage studies. Furthermore, determination of the charge carrier conduction model, charge carrier mobility, density of the trap states, density of the available charge carrier, free-carrier concentration, effective density of states in the conduction band, activation energy of the carrier transport, as well as ion hopping was successfully conducted for the ReO3@ReS2 based on the experimental data. -

United States Patent Office
Patented Feb. 10, 1948 2,435,566 UNITED STATES PATENT OFFICE 2.435.566 ' PEROXIDE BLEACHING 0F GROUND woon Daniel 0. Adams and George B. Hughey, ton, Va... assignors to West Virginia PulpCoving and Paper Company, New York, N. Y., a corporation of Delaware No Drawing. Application" October 16, 1944, Serial No. 558.951 ' 3 Claims. (01. 8-—104) Our invention relates to the bleaching of 2 ground wood, more especially groundloak and Example I other ground woods such as chestnut, chestnut A 14.8 gram sample of red oak ground wood in oak, maple, etc., which contain substantial the form of a slurry or 5% oven dried (0. D.) amounts of tannin compounds and other natural color bodies. consistency was extracted for one hour at 77° F. In recent with an NaOH solution at a. concentration oi.’ 1 years much study has been given to gram per liter. At the end of one hour the'pulp ' the bleaching of ground wood. Usually and espe was thickened and thoroughly washed. The ex cially for the manufacture of newsprint, ground tracted pulp was then treated with a quantity wood is not bleached. However, the desirable 10 of sodium peroxide equal to 3.5% of the weight printing characteristics of paper containing a of the unbleached pulp on a dry basis, 1. e., .525 large proportion of ground wood have led'to pro gram. The pulp was in the form of a slurry oi’ posals for its use in high grades of paper such 5% consistency at a temperature of 92° F. -

List of Reactive Chemicals
LIST OF REACTIVE CHEMICALS Chemical Prefix Chemical Name Reactive Reactive Reactive CAS# Chemical Chemical Chemical Stimulus 1 Stimulus 2 Stimulus 3 111-90-0 "CARBITOL" SOLVENT D 111-15-9 "CELLOSOLVE" ACETATE D 110-80-5 "CELLOSOLVE" SOLVENT D 2- (2,4,6-TRINITROPHENYL)ETHYL ACETATE (1% IN ACETONE & BENZENE S 12427-38-2 AAMANGAN W 88-85-7 AATOX S 40487-42-1 AC 92553 S 105-57-7 ACETAL D 75-07-0 ACETALDEHYDE D 105-57-7 ACETALDEHYDE, DIETHYL ACETAL D 108-05-4 ACETIC ACID ETHENYL ESTER D 108-05-4 ACETIC ACID VINYL ESTER D 75-07-0 ACETIC ALDEHYDE D 101-25-7 ACETO DNPT T 126-84-1 ACETONE DIETHYL ACETAL D 108-05-4 ACETOXYETHYLENE D 108-05-4 1- ACETOXYETHYLENE D 37187-22-7 ACETYL ACETONE PEROXIDE, <=32% AS A PASTE T 37187-22-7 ACETYL ACETONE PEROXIDE, <=42% T 37187-22-7 ACETYL ACETONE PEROXIDE, >42% T S 644-31-5 ACETYL BENZOYL PEROXIDE (SOLID OR MORE THAN 45% IN SOLUTION) T S 644-31-5 ACETYL BENZOYL PEROXIDE, <=45% T 506-96-7 ACETYL BROMIDE W 75-36-5 ACETYL CHLORIDE W ACETYL CYCLOHEXANE SULFONYL PEROXIDE (>82% WITH <12% WATER) T S 3179-56-4 ACETYL CYCLOHEXANE SULFONYL PEROXIDE, <=32% T 3179-56-4 ACETYL CYCLOHEXANE SULFONYL PEROXIDE, <=82% T 674-82-8 ACETYL KETENE (POISON INHALATION HAZARD) D 110-22-5 ACETYL PEROXIDE, <=27% T 110-22-5 ACETYL PEROXIDE, SOLID, OR MORE THAN 27% IN SOLUTION T S 927-86-6 ACETYLCHOLINE PERCHLORATE O S 74-86-2 ACETYLENE D 74-86-2 ACETYLENE (LIQUID) D ACETYLENE SILVER NITRATE D 107-02-08 ACRALDEHYDE (POISON INHALATION HAZARD) D 79-10-7 ACROLEIC ACID D 107-02-08 ACROLEIN, INHIBITED (POISON INHALATION HAZARD) D 107-02-08 ACRYLALDEHYDE (POISON INHALATION HAZARD) D 79-10-7 ACRYLIC ACID D 141-32-2 ACRYLIC ACID BUTYL ESTER D 140-88-5 ACRYLIC ACID ETHYL ESTER D 96-33-3 ACRYLIC ACID METHYL ESTER D Stimulus - Stimuli is the thermal, physical or chemical input needed to induce a hazardous reaction. -
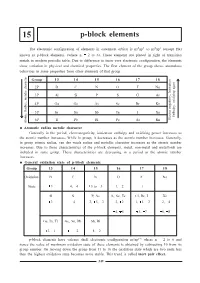
P-Block Elements
15 p-block elements The electronic configuration of elements in outermost orbital is ns2np1 to ns2np6 (except He) known as p-block elements. (where n = 2 to 6). These elements are placed in right of transition metals in modern periodic table. Due to difference in inner core electronic configuration, the elements show variation in physical and chemical properties. The first element of the group shows anomalous behaviour in some properties from other elements of that group. Group 13 14 15 16 17 18 2P BC NO F Ne 3P Al Si P S Cl Ar , Ionisation 4P Ga Ge As Se Br Kr , oxidizing agent 5P In Sn Sb Te I Xe Enthalpy 6P Tl Pb Bi Po At Rn Electro negativity Atomic Radius, metalic character Atomatic radius metalic character Generally in the period, electronegativity, ionization enthalpy and oxidising power increases as the atomic number increases. While in group, it decreases as the atomic number increases. Generally, in group atomic radius, van der waals radius and metallic character increases as the atomic number increases. Due to these characteristics of the p-block elements, metal, non-metal and metalloids are included in same group. These characteristics are decreasing in a period as the atomic number increases. General oxidation state of p-block elements Group 13 14 15 16 17 18 Oxidation BC NO F Ne State +3 +4, -4 +5 to -3 -1, -2 -1 - Al Si P, As S, Se, Te Cl, Br, I Xe +3 +4 +3, +5, -3 -2, +2 -1, +1, +3 +2, +4 +4, +6 +5, +7 +6, +8 Ga, In, Tl Ge, Sn, Pb Sb, Bi +3, +1 +4, +2 +4, +2 p-block elements have valence shell electronic configuration ns2np16 where n = 2 to 6 and hence the value of maximum oxidation state of these elements is obtained by subtracting 10 from its group number. -

United States Patent (19) 11 4,399,633 Haughey Et Al
United States Patent (19) 11 4,399,633 Haughey et al. 45) Aug. 23, 1983 PRODUCTION OF ALKAL METAL, OR 3,786,573 1/1974 Scheppe et al. ........................ 34/10 (54 ALKALINE EARTH METAL PEROXDES 3,789,513 2/1974 Mark ............... ... 34/10 3,879,856 4/1975 Barr......................................... 34/10 75 Inventors: Douglas P. Haughey, Chester; Malcolm H. Millar, Liverpool, both FOREIGN PATENT DOCUMENTS of England 52-69711 6/1977 Japan .................................... 47/57.6 73 Assignee: Interox Chemicals Limited, London, 799251 8/1958 United Kingdom.................... 34/11 England OTHER PUBLICATIONS 21) Appl. No.: 252,323 Perry, Chemical Engineers' Handbook, Third Edition, 22 Filed: Apr. 9, 1981 McGraw-Hill Book Co., Inc. (1950), pp. 834-838. (51) Int. Cl’................................................ CO1D 1/02 Primary Examiner-O. R. Vertiz (52) U.S.C. .......................................... 47/57.6; 34/9; Assistant Examiner-Wayne A. Langel 34/10, 34/11; 71/63; 71/77; 423/582; 42.3/583; Attorney, Agent, or Firm-Larson and Taylor 423/265 57 ABSTRACT (58) Field of Search ...................... 423/582,583; 34/9, Alkali metal or alkaline earth metal peroxides, for exam 34/10, 11; 71/63, 64.03, 904, 77; 47/57.6; 119/1 ple, particularly, calcium peroxide, are produced by (56) References Cited recovering a moist peroxide product from an aqueous U.S. PATENT DOCUMENTS mixture, formed by reacting hydrogen peroxide and a 1,308,942 7/1919 French .................................... 34/11 hydroxide or oxide of the alkali metal or alkaline earth 2,290,862 7/1942 Canning . 423/583 metal, and drying the moist product by means of a pneu 2,350,162 5/1944 Gordon ......... -

Ternary and Quaternary Phases in the Alkali-Earth - Rhenium - Oxygen System
Ternary and quaternary phases in the alkali-earth - rhenium - oxygen system Vom Fachbereich Material- und Geowissenschaften der Technischen Universität Darmstadt zur Erlangung des akademischen Grades eines Doktor rer. nat. genehmigte Dissertation angefertigt von Dipl.-Chem. Kirill G. Bramnik Berichterstatter: Prof. Dr. H. Fuess Mitberichterstatter: Prof. Dr. J. Galy Prof. Dr. H.M. Ortner Tag der Einreichung: 09.05.2001 Tag der mündlichen Prüfung: 07.06.2001 Darmstadt 2001 D 17 2 Моим родителям 3 1 INTRODUCTION ............................................................................................. 5 2 LITERATURE SURVEY.................................................................................. 7 2.1 STRUCTURAL CHEMISTRY OF TERNARY RHENIUM OXIDES................................. 7 2.2 SYNTHETIC PROBLEMS.................................................................................... 24 2.3 MAGNETIC AND ELECTRICAL PROPERTIES OF COMPLEX RHENIUM OXIDES ...... 26 3 EXPERIMENTAL........................................................................................... 30 3.1 POWDER DIFFRACTION.................................................................................... 30 3.1.1 X-ray powder diffraction...............................................................................................30 3.1.2 Neutron powder diffraction...........................................................................................30 3.2 ELECTRON DIFFRACTION................................................................................ -

Reaction Mechanism and Process Control of Hydrogen Reduction of Ammonium Perrhenate
metals Article Reaction Mechanism and Process Control of Hydrogen Reduction of Ammonium Perrhenate Junjie Tang 1,2, Yuan Sun 2,*, Chunwei Zhang 2,3, Long Wang 2, Yizhou Zhou 2,*, Dawei Fang 3 and Yan Liu 4 1 School of Biomedical & Chemical Engineering, Liaoning Institute of Science and Technology, Benxi 117004, China; [email protected] 2 Superalloys research department, Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China; [email protected] (C.Z.); [email protected] (L.W.) 3 College of chemistry, Liaoning University, Shenyang 110036, China; davidfi[email protected] 4 Key Laboratory for Ecological Utilization of Multimetallic Mineral, Ministry of Education, Northeastern University, Shenyang 110819, China; [email protected] * Correspondence: [email protected] (Y.S.); [email protected] (Y.Z.); Tel.: +86-024-23971767 (Y.S.); +86-024-83978068 (Y.Z.) Received: 17 April 2020; Accepted: 13 May 2020; Published: 15 May 2020 Abstract: The preparation of rhenium powder by a hydrogen reduction of ammonium perrhenate is the only industrial production method. However, due to the uneven particle size distribution and large particle size of rhenium powder, it is difficult to prepare high-density rhenium ingot. Moreover, the existing process requires a secondary high-temperature reduction and the deoxidization process is complex and requires a high-temperature resistance of the equipment. Attempting to tackle the difficulties, this paper described a novel process to improve the particle size distribution uniformity and reduce the particle size of rhenium powder, aiming to produce a high-density rhenium ingot, and ammonium perrhenate is completely reduced by hydrogen at a low temperature. -

CHO-DISSERTATION-2020.Pdf
Copyright by Shin Hum Cho 2020 The Dissertation Committee for Shin Hum Cho Certifies that this is the approved version of the following Dissertation: Infrared Plasmonic Doped Metal Oxide Nanocubes Committee: Delia J. Milliron, Supervisor Brian A. Korgel Thomas M. Truskett Xiaoqin Elaine Li Infrared Plasmonic Doped Metal Oxide Nanocubes by Shin Hum Cho Dissertation Presented to the Faculty of the Graduate School of The University of Texas at Austin in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy The University of Texas at Austin May 2020 Abstract Infrared Plasmonic Doped Metal Oxide Nanocubes Shin Hum Cho, Ph.D. The University of Texas at Austin, 2020 Supervisor: Delia J. Milliron Localized surface plasmon resonance (LSPR) in semiconductor nanocrystals (NCs) that results in resonant absorption, scattering, and near field enhancement around the NC can be tuned across a wide optical spectral range from visible to far-infrared by synthetically varying doping level. Cube-shaped NCs of conventional metals like gold and silver generally exhibit LSPR in the visible region with spectral modes determined by their faceted shapes. However, faceted NCs exhibiting LSPR response in the infrared (IR) region are relatively rare. We describe the colloidal synthesis of nanoscale fluorine- doped indium oxide (F:In2O3) cubes with LSPR response in the IR region, wherein fluorine was found to both direct the cubic morphology and act as an aliovalent dopant. The presence of fluorine was found to impart higher stabilization to the (100) facets, suggesting that the cubic morphology results from surface binding of F-atoms. In addition, fluorine acts as an anionic aliovalent dopant in the cubic bixbyite lattice of In2O3, introducing a high concentration of free electrons leading to LSPR.