AACT Herbal Dietary Supplements SIG Abstracts July 2017
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
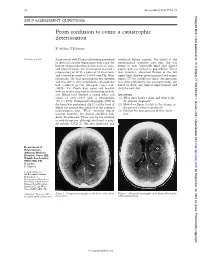
From Confusion to Coma: a Catastrophic Deterioration
52 Postgrad Med J 2001;77:52–55 Postgrad Med J: first published as 10.1136/pmj.77.903.53a on 1 January 2001. Downloaded from SELF ASSESSMENT QUESTIONS From confusion to coma: a catastrophic deterioration K Ashkan, F Johnston Answers on p 56. A previously well 45 year old woman presented ventilated before transfer. On arrival at the to the local casualty department with a one day neurosurgical intensive care unit, she was history of generalised headache, neck stiVness, found to have bilaterally fixed and dilated and blurred vision. On examination she had a pupils, with no corneal or gag reflexes. There temperature of 38°C, a pulse of 80 beats/min, was, however, abnormal flexion of the left and a blood pressure of 130/80 mm Hg. Neu- upper limb. She was given mannitol and urgent rologically, she had spontaneous eye opening repeat CT was carried out (fig 2). An operation and was able to obey commands, although she was then performed, but postoperatively she had confused speech (Glasgow coma scale failed to show any clinical improvement and (GCS), 14). Pupils were equal and reactive died the next day. with no cranial or peripheral neurological defi- cits. Blood tests showed a raised white cell Questions count of 20.9 × 109/l with a neutrophilia (1) What does figure 1 show and what is the (19.2 × 109/l). Computed tomography (CT) of diVerential diagnosis? the brain was performed (fig 1), on the basis of (2) How does figure 2 relate to the change in which the patient was referred to the regional the patient’s clinical condition? neurosurgical unit. -

Hypervitaminosis - an Emerging Pathological Condition
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Review Article Hypervitaminosis - An Emerging Pathological Condition J K Roop PG Department of Zoology, JC DAV College (Affiliated to Panjab University, Chandigarh), Dasuya-144205, District Hoshiarpur, Punjab, India ABSTRACT Vitamins are essential organic compounds that are required in small amounts to regulate various metabolic activities in the body. Prolonged and overconsumption of pharmaceutical forms of both water-soluble and fat-soluble vitamins may lead to toxicity and/or hypervitaminosis. Hypervitaminosis is an acute emerging pathological condition of the body due to excess accumulation of any of the vitamins. In case of acute poisoning with vitamin supplements/drugs, emergency assistance is required to detoxify the effects and restore the organization, structure and function of body’s tissues and organs. Sometimes death may occur due to intoxication to liver, kidney and heart. So, to manage any type of hypervitaminosis, proper diagnosis is essential to initiate eliminating the cause of its occurrence and accelerate the elimination of the supplement from the body. The present review discusses the symptoms of hypervitaminosis that seems to be a matter of concern today and management strategies to overcome toxicity or hypervitaminosis. Keywords: Hypervitaminosis, Toxicity, Vitamins, Vitamin pathology, Fat-soluble vitamin, Water- soluble vitamin. INTRODUCTION body, particularly in the liver. Vitamin B Vitamins are potent organic Complex and vitamin C are water- soluble. compounds present in small concentrations They are dissolved easily in food during in various fruits and vegetables. They cooking and a portion of these vitamins may regulate physiological functions and help in be destroyed by heating. -

Question of the Day Archives: Monday, December 5, 2016 Question: Calcium Oxalate Is a Widespread Toxin Found in Many Species of Plants
Question Of the Day Archives: Monday, December 5, 2016 Question: Calcium oxalate is a widespread toxin found in many species of plants. What is the needle shaped crystal containing calcium oxalate called and what is the compilation of these structures known as? Answer: The needle shaped plant-based crystals containing calcium oxalate are known as raphides. A compilation of raphides forms the structure known as an idioblast. (Lim CS et al. Atlas of select poisonous plants and mushrooms. 2016 Disease-a-Month 62(3):37-66) Friday, December 2, 2016 Question: Which oral chelating agent has been reported to cause transient increases in plasma ALT activity in some patients as well as rare instances of mucocutaneous skin reactions? Answer: Orally administered dimercaptosuccinic acid (DMSA) has been reported to cause transient increases in ALT activity as well as rare instances of mucocutaneous skin reactions. (Bradberry S et al. Use of oral dimercaptosuccinic acid (succimer) in adult patients with inorganic lead poisoning. 2009 Q J Med 102:721-732) Thursday, December 1, 2016 Question: What is Clioquinol and why was it withdrawn from the market during the 1970s? Answer: According to the cited reference, “Between the 1950s and 1970s Clioquinol was used to treat and prevent intestinal parasitic disease [intestinal amebiasis].” “In the early 1970s Clioquinol was withdrawn from the market as an oral agent due to an association with sub-acute myelo-optic neuropathy (SMON) in Japanese patients. SMON is a syndrome that involves sensory and motor disturbances in the lower limbs as well as visual changes that are due to symmetrical demyelination of the lateral and posterior funiculi of the spinal cord, optic nerve, and peripheral nerves. -

Nutrition Journal of Parenteral and Enteral
Journal of Parenteral and Enteral Nutrition http://pen.sagepub.com/ Micronutrient Supplementation in Adult Nutrition Therapy: Practical Considerations Krishnan Sriram and Vassyl A. Lonchyna JPEN J Parenter Enteral Nutr 2009 33: 548 originally published online 19 May 2009 DOI: 10.1177/0148607108328470 The online version of this article can be found at: http://pen.sagepub.com/content/33/5/548 Published by: http://www.sagepublications.com On behalf of: The American Society for Parenteral & Enteral Nutrition Additional services and information for Journal of Parenteral and Enteral Nutrition can be found at: Email Alerts: http://pen.sagepub.com/cgi/alerts Subscriptions: http://pen.sagepub.com/subscriptions Reprints: http://www.sagepub.com/journalsReprints.nav Permissions: http://www.sagepub.com/journalsPermissions.nav >> Version of Record - Aug 27, 2009 OnlineFirst Version of Record - May 19, 2009 What is This? Downloaded from pen.sagepub.com by Karrie Derenski on April 1, 2013 Review Journal of Parenteral and Enteral Nutrition Volume 33 Number 5 September/October 2009 548-562 Micronutrient Supplementation in © 2009 American Society for Parenteral and Enteral Nutrition 10.1177/0148607108328470 Adult Nutrition Therapy: http://jpen.sagepub.com hosted at Practical Considerations http://online.sagepub.com Krishnan Sriram, MD, FRCS(C) FACS1; and Vassyl A. Lonchyna, MD, FACS2 Financial disclosure: none declared. Preexisting micronutrient (vitamins and trace elements) defi- for selenium (Se) and zinc (Zn). In practice, a multivitamin ciencies are often present in hospitalized patients. Deficiencies preparation and a multiple trace element admixture (containing occur due to inadequate or inappropriate administration, Zn, Se, copper, chromium, and manganese) are added to par- increased or altered requirements, and increased losses, affect- enteral nutrition formulations. -

Pharmacology
STATE ESTABLISHMENT «DNIPROPETROVSK MEDICAL ACADEMY OF HEALTH MINISTRY OF UKRAINE» V.I. MAMCHUR, V.I. OPRYSHKO, А.А. NEFEDOV, A.E. LIEVYKH, E.V.KHOMIAK PHARMACOLOGY WORKBOOK FOR PRACTICAL CLASSES FOR FOREIGN STUDENTS STOMATOLOGY DEPARTMENT DNEPROPETROVSK - 2016 2 UDC: 378.180.6:61:615(075.5) Pharmacology. Workbook for practical classes for foreign stomatology students / V.Y. Mamchur, V.I. Opryshko, A.A. Nefedov. - Dnepropetrovsk, 2016. – 186 p. Reviewed by: N.I. Voloshchuk - MD, Professor of Pharmacology "Vinnitsa N.I. Pirogov National Medical University.‖ L.V. Savchenkova – Doctor of Medicine, Professor, Head of the Department of Clinical Pharmacology, State Establishment ―Lugansk state medical university‖ E.A. Podpletnyaya – Doctor of Pharmacy, Professor, Head of the Department of General and Clinical Pharmacy, State Establishment ―Dnipropetrovsk medical academy of Health Ministry of Ukraine‖ Approved and recommended for publication by the CMC of State Establishment ―Dnipropetrovsk medical academy of Health Ministry of Ukraine‖ (protocol №3 from 25.12.2012). The educational tutorial contains materials for practical classes and final module control on Pharmacology. The tutorial was prepared to improve self-learning of Pharmacology and optimization of practical classes. It contains questions for self-study for practical classes and final module control, prescription tasks, pharmacological terms that students must know in a particular topic, medical forms of main drugs, multiple choice questions (tests) for self- control, basic and additional references. This tutorial is also a student workbook that provides the entire scope of student’s work during Pharmacology course according to the credit-modular system. The tutorial was drawn up in accordance with the working program on Pharmacology approved by CMC of SE ―Dnipropetrovsk medical academy of Health Ministry of Ukraine‖ on the basis of the standard program on Pharmacology for stomatology students of III - IV levels of accreditation in the specialties Stomatology – 7.110105, Kiev 2011. -

THE MINISTRY of HEALTH of UKRAINE NATIONAL UNIVERSITY of PHARMACY KROK WITHOUT PROBLEMS Study Guide for License Test Preparing
THE MINISTRY OF HEALTH OF UKRAINE NATIONAL UNIVERSITY OF PHARMACY THE DEPARTMENT OF PHARMACOLOGY Shtrygol’ S.Yu., Vereitinova V.P., Kudina O.V., Kononenko A.V. KROK WITHOUT PROBLEMS Study guide for license test preparing «Krok-1 Pharmacy» Kharkov-2019 Contents General pharmacology ............... 4 37. Pyracetam 38 80. Vicasol 59 Medicines affecting the respiratory system ................................................................................................................................................... 38 Medicines ........................... 10 81. Heparin 59 38. Acetylcysteine 38 Medicines affecting afferent 82. Dicumarol 61 39. Glaucin 39 Vitamin-containing medicines ....................................................................................................................................................................... 61 innervations: ............................. 10 Local anesthetics ................................................................40. Ambroxol................................ ................................41 83................................. Retinol .......................... 10 61 1. Lidocain 10 41. Theophylline 41 84. Alpha-tocopherol 62 2. Novocaine 11 42. Cromoline sodium 41 85. Ascorutinum 62 Medicines affecting efferent Cardiotonic medicines ................................................................86. Pyridoxine................................ ................................63 .................................................. 41 43. Digoxin 41 innervations: ............................ -

Vitamin D Toxicity
Post N Med 2016; XXIX(10): 756-759 ©Borgis *Ewa Marcinowska-Suchowierska1, Paweł Płudowski2 Vitamin D toxicity Zatrucie witaminą D 1Department of Geriatric, Internal Medicine and Metabolic Bone Disease, Centre of Postgraduate Medical Education, Warsaw Head of Department: Professor Marek Tałałaj, MD, PhD 2Department of Biochemistry, Radioimmunology and Experimental Medicine, The Children’s Memorial Health Institute, Warsaw Head of Department: Professor Roman Janas, PhD Keywords Summary vitamin D, 25(OH)D, toxicity, clinical Vitamin D toxicity (VDT), also called hypervitaminosis D, is a rare but potentially serious symptoms and management condition that occurs when an individual has excessive 25(OH)D levels in the bloodstream. Vitamin D toxicity is usually caused by extremely high doses of vitamin D supplements, not by diet or skin exposure to the sun. The main clinical consequence of VDT is elevated Słowa kluczowe serum calcium level (hypercalcemia) and a variety of nonspecific symptoms. The literature witamina D, 25(OH)D, toksyczność, reports that hypercalcemia due to overdosed vitamin D may appear if serum 25(OH)D objawy kliniczne, leczenie levels are higher than 150-200 ng/ml. Many different mechanisms have been proposed to account for VDT, including the vitamin D metabolite itself, VDR number, activity of 1 al- fa-hydroxylase, inhibition of vitamin D catabolism, and the capacity of VDBP. Mounting evidence that higher levels of vitamin D may have beneficial effects on bone and cellular health may predispose to enhanced administration of vitamin D and increased frequency of VDT. Hypercalcemia from VDT is rare, but a dangerous state for the organism and should receive adequate and sensible treatment. -

Principles and Methods for the Assessment of Neurotoxicity Associated with Exposure to Chemicals
lnkr national Environmental Health Criteria 60 Principles and Methods for the Assessment of Neurotoxicity Associated With Exposure to Chemicals Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization WORLD HEALTH ORGANIZATION GENEVA 1986 Other titles available in the ENVIRONMENTAL HEALTH CRITERIA series include: 1. Mercury 32. Methylene Chloride 2. Polychlorinated Biphenyls and 33. Epichlorohydrin Terphenyls 34. Chlordane 3. Lead 35. Extremely Low Frequency 4. Oxides of Nitrogen (ELF) Fields 5. Nitrates, Nitrites, and 36. Fluorides and Fluorine N-Nitroso Compounds 37. Aquatic (Marine and 6. Principles and Methods for Freshwater) Biotoxins Evaluating the Toxicity of 38. Heptachlor Chemicals, Part 1 39. Paraquat and Diquat 7. Photochemical Oxidants 40. Endosulfan 8. Sulfur Oxides and Suspended 41. Quintozene Particulate Matter 42. Tecnazene 9. DDT and its Derivatives 43. Chlordecone 10. Carbon Disulfide 44. Mirex 11. Mycotoxins 45. Camphechlor 12. Noise 46. Guidelines for the Study of 13. Carbon Monoxide Genetic Effects in Human 14. Ultraviolet Radiation Populations 15. Tin and Organotin Compounds 47. Summary Report on the 16. Radiofrequency and Microwaves Evaluation of Short-Term Tests 17. Manganese for Carcinogens (Collaborative 18. Arsenic Study on In Vitro Tests) 19. Hydrogen Sulfide 48. Dimethyl Sulfate 20. Selected Petroleum Products 49. Acrylamide 21. Chlorine and Hydrogen 50. Trichloroethylene Chloride 51. Guide to Short-Term Tests for 22. Ultrasound Detecting Mutagenic and 23. Lasers and Optical Radiation Carcinogenic Chemicals 24. Titanium 52. Toluene 25. Selected Radionuclides 53. Asbestos and Other Natural 26. Styrene Mineral Fibres 27. Guidelines on Studies in 54. Ammonia Environmental Epidemiology 55. -

Graduate School of Biomedical Sciences
Graduate School of Biomedical Sciences 32nd Annual Student Research Week March 10-13, 2020 Texas Tech University Health Sciences Center (TTUHSC) Lubbock, Texas The Graduate School of Biomedical Sciences 2020 Student Research Week Committee Director: Bradley Schniers Vice Director of Marketing: Mariacristina Mazzitelli Vice Director of Poster Competition: Rachel Washburn Vice Director of Operations & Judging: Ryan Sweazey Website design and maintenance: Danny Boren, Graduate School of Biomedical Sciences Communications and social media: Suzanna Cisneros and Amy Skousen, Office of Communications Marketing; Leslie Fowler, Graduate School of Biomedical Sciences Speaker travel arrangements: Leslie Fowler, Graduate School of Biomedical Sciences Abstract book design: Deidra Satterwhite, Office of Student Life Student Research Week Banquet: Korac K, Graduate School of Biomedical Sciences Graduate Student Association; Velia Martinez, Graduate School of Biomedical Sciences The 2020 Student Research Week Committee would like to extend their warmest thanks to the following for their contributions and support in making Student Research Week a great success this year: The Graduate School of Biomedical Sciences staff: Leslie Fowler, Pam Johnson, Ashlee Rigsby and Velia Martinez The Office of Student Life: Deidra Satterwhite The Office of Communications and Marketing: Suzanna Cisneros, Amy Skousen and Kami Hunt The Office of the President: Bryce Looney The School of Medicine Office of the Dean: Charity Donaldson Educational Media Services: Neal Hinkle The departments of cell biology and biochemistry, pharmacology and neuroscience, immunology and molecular microbiology, cell physiology and molecular biophysics, medical education and graduate medical education; Graduate School of Biomedical Sciences at Lubbock, Abilene, and Amarillo, the School of Medicine, the School of Nursing, the School of Health Professions, the School of Pharmacy, the Office of Interprofessional Education, and Texas Tech University. -

The Vitamin B6 Paradox Supplementation with High
Toxicology in Vitro 44 (2017) 206–212 Contents lists available at ScienceDirect Toxicology in Vitro journal homepage: www.elsevier.com/locate/toxinvit The vitamin B6 paradox: Supplementation with high concentrations of MARK pyridoxine leads to decreased vitamin B6 function ⁎ Misha F. Vrolijka, , Antoon Opperhuizena,b, Eugène H.J.M. Jansenc, Geja J. Hagemana, Aalt Basta, Guido R.M.M. Haenena a Department of Pharmacology and Toxicology, Maastricht University, Maastricht, The Netherlands b Netherlands Food and Consumer Product Safety Authority (NVWA), Utrecht, The Netherlands c National Institute for Public Health and the Environment (RIVM), Bilthoven, The Netherlands ARTICLE INFO ABSTRACT Keywords: Vitamin B6 is a water-soluble vitamin that functions as a coenzyme in many reactions involved in amino acid, Vitamin B6 carbohydrates and lipid metabolism. Since 2014, > 50 cases of sensory neuronal pain due to vitamin B6 sup- Neuropathy plementation were reported. Up to now, the mechanism of this toxicity is enigmatic and the contribution of the Pyridoxine various B6 vitamers to this toxicity is largely unknown. Supplements In the present study, the neurotoxicity of the different forms of vitamin B6 is tested on SHSY5Y and CaCo-2 Neurotoxic cells. Cells were exposed to pyridoxine, pyridoxamine, pyridoxal, pyridoxal-5-phosphate or pyridoxamine-5- phosphate for 24 h, after which cell viability was measured using the MTT assay. The expression of Bax and caspase-8 was tested after the 24 h exposure. The effect of the vitamers on two pyridoxal-5-phosphate dependent enzymes was also tested. Pyridoxine induced cell death in a concentration-dependent way in SHSY5Y cells. The other vitamers did not affect cell viability. -

Iatrogenic Vitamin D Overdose Resulting in Acute Pancreatitis with Acute Kidney Injury
Open Access http://www.jparathyroid.com Journal of Journal of Parathyroid Disease 2018,6(1),29–31 Case Report DOI: 10.15171/jpd.2018.10 Iatrogenic vitamin D overdose resulting in acute pancreatitis with acute kidney injury Rajesh Singh1, Manish R Balwani2*, Umesh Godhani3, Pravin Ghule4, Priyanka Tolani5, Vivek Kute6 Abstract Vitamin D deficiency is prevalent worldwide. Most of patients with mild vitamin D deficiency are often over-treated with higher dose of vitamin D supplementation than required resulting in toxic levels of vitamin D. While correcting, one should regularly look for adverse effects of overcorrection resulting in hypervitaminosis D. We discuss here a similar case of 34-year-old woman who presented to us with persistent acute abdominal pain since 48 hours and oliguria since 24 hours. She was found to have hypercalcemia induced pancreatitis with acute kidney injury. Cause of hypercalcemia was found to be hypervitaminosis D. Thereafter, she was treated with aggressive hydration and diuretics, and required calcitonin for control of hypercalcemia to which she responded. Thus we suggest that in any patient who presents with hypercalcemia with low parathyroid levels, hypervitaminosis D should be suspected. Primary care physicians should be alerted of such cases, to avoid overcorrection of vitamin D. Mild vitamin D deficiency should be initially corrected with adequate sunlight exposure and fortified/enriched vitamin D food supplements. Keywords: Hypercalcemia, Hypervitaminosis D, Acute kidney injury, Acute pancreatitis Please cite this paper as: Singh R, Balwani MR, Godhani U, Ghule P, Tolani P, Kute V. Iatrogenic vitamin D overdose resulting in acute pancreatitis with acute kidney injury. -

16-08-2011 Nin Scientific Publications 1919 – 2011 1919
16-08-2011 NIN SCIENTIFIC PUBLICATIONS 1919 – 2011 1919 McCarrison, R. : THE GENESIS OF OEDEMA IN BERI-BERI. Proc R Soc BRes. 91:103-110, 1919. McCarrison, R. : THE PATHOGENESIS OF DEFICIENCY DISEASE - I. IndianJ Med Res. 6:275-355, 1919. McCarrison, R. : THE PATHOGENESIS OF DEFICIENCY DISEASE - II THEEFFECTS OF DEPRIVATION OF `B' ACCESSORY FOOD FACTORS. Indian J MedRes. 6:550-556, 1919. McCarrison, R. : INVOLUTION OF THE THYMUS IN BIRDS. Indian J MedRes. 6:557-559, 1919. McCarrison, R. : THE PATHOGENESIS OF DEFICIENCY DISEASE - III THEINFLUENCE OF DIETARIES DEFICIENT IN ACCESSARY FOOD FACTORS ON THEINTESTINE. Indian J Med Res. 7:167-187, 1919. McCarrison, R. : THE PATHOGENESIS OF DEFICIENCY DISEASE - IV THEINFLUENCE OF SCORBUTIE DIET ON THE ADRENAL GLANDS. Indian J Med Res. 7:188-194, 1919. McCarrison, R. : THE PATHOGENESIS OF DEFICIENCY DISEASE - VHISTO- PATHOLOGY. Indian J Med Res. 7:269-278, 1919. McCarrison, R. : THE PATHOGENESIS OF DEFICIENCY DISEASES - VI THEINFLUENCE OF A SCORBUTIC DIET ON THE BLADDER. Indian J Med Res. 7:279-282, 1919. McCarrison, R. : THE PATHOGENESIS OF DEFICIENCY DISEASE - VII THEEFFECTS OF AUTOCLAVED RICE DIETARIES ON THE GASTRO- INTESTINAL TRACTOF MONKEYS. Indian J Med Res. 7:283-307, 1919. McCarrison, R. : THE PATHOGENESIS OF DEFICIENCY DISEASE - VIII THEGENERAL EFFECT OF DEFICIENT DIETARIES ON MONKEYS. Indian J Med Res. 7:308-341, 1919. McCarrison, R. : THE PATHOGENESIS OF DEFICIENCY DISEASE - IX ON THEOCCURRENCE OF RECENTLY DEVELOPED CANCER OF THE STOMACH IN A MONKEYFED ON FOOD DEFICIENT IN VITAMINS. Indian J Med Res. 7:342-345, 1919. McCarrison, R. : THE INFLUENCE OF DEFICIENCY OF ACCESSARY FOODFACTORS ON THE INTESTINE.