Nutrition Journal of Parenteral and Enteral
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Polymorphisms in Folic Acid Metabolism Genes Do Not Associate with Cancer Cachexia in Japanese Gastrointestinal Patients
ORIGINAL PAPER Nagoya J. Med. Sci. 80. 529–539, 2018 doi:10.18999/nagjms.80.4.529 Polymorphisms in folic acid metabolism genes do not associate with cancer cachexia in Japanese gastrointestinal patients Takuto Morishita1, Asahi Hishida1, Yoshinaga Okugawa2,3,6, Yuuki Morimoto2, Yumiko Shirai4, Kyoko Okamoto5, Sachiko Momokita6, Aki Ogawa5, Koji Tanaka2,7, Ryutaro Nishikawa2, Yuji Toiyama7, Yasuhiro Inoue7, Hiroyuki Sakurai8, Hisashi Urata2, Motoyoshi Tanaka3, and Chikao Miki2,7 1Department of Preventive Medicine, Nagoya University Graduate School of Medicine, Nagoya, Japan 2Departments of Surgery, Iga City General Hospital, Iga, Japan 3Departments of Medical Oncology, Iga City General Hospital, Iga, Japan 4Departments of Nutrition, Iga City General Hospital, Iga, Japan 5Departments of Nursing, Iga City General Hospital, Iga, Japan 6Departments of Biochemical Laboratory, Iga City General Hospital, Iga, Japan 7Department of Gastrointestinal and Pediatric Surgery, Division of Reparative Medicine, Institute of Life Sciences, Mie University Graduate School of Medicine, Tsu, Japan 8Department of Hepatobiliary Pancreatic and Transplant Surgery, Mie University Graduate School of Medicine, Tsu, Japan. ABSTRACT We used clinical data from Iga General Hospital to examine the association between polymorphisms in MTR (methionine synthase) A2756G (rs1805087), MTRR (methionine synthase reductase) His595Tyr (rs10380), MTHFR (methylenetetrahydrofolate reductase) C677T (rs1801133), MTHFR A1298C (rs1801131) and SHMT (serine hydroxymethyltransferase) C1420T (rs1979277), which are genes involved in folate metabolism, and the risk of weight loss in patients with gastrointestinal cancers, with the aim of establishing personalized palliative care for each patient based on genetic information. The data from 59 patients (37 males and 22 females) with gastrointestinal cancers who visited the outpatient clinic for cancer chemotherapy and palliative care at Iga General Hospital from December 2011 to August 2015 were analyzed. -

Dysmagnesemia in Covid-19 Cohort Patients: Prevalence and Associated Factors
Magnesium Research 2020; 33 (4): 114-122 ORIGINAL ARTICLE Dysmagnesemia in Covid-19 cohort patients: prevalence and associated factors Didier Quilliot1, Olivier Bonsack1, Roland Jaussaud2, Andre´ Mazur3 1 Transversal Nutrition Unit and; 2 Internal Medicine and Clinical Immunology. Nancy University Hospital, University of Lorraine, France; 3 Universite´ Clermont Auvergne, INRAE, UNH, Unite´ de Nutrition Humaine, Clermont-Ferrand, France Correspondence <[email protected]> Abstract. Hypomagnesemia and hypermagnesemia could have serious implications and possibly lead to progress from a mild form to a severe outcome of Covid-19. Susceptibility of subjects with low magnesium status to develop and enhance this infection is possible. There is little data on the magnesium status of patients with Covid-19 with different degrees of severity. This study was conducted to evaluate prevalence of dysmagnesemia in a prospective Covid-19 cohort study according to the severity of the clinical manifestations and to identify factors associated. Serum magnesium was measured in 300 of 549 patients admitted to the hospital due to severe Covid-19. According to the WHO guidelines, patients were classified as moderate, severe, or critical. 48% patients had a magnesemia below 0.75 mmol/L (defined as magnesium deficiency) including 13% with a marked hypomagnesemia (<0.65 mmol/L). 9.6% had values equal to or higher than 0.95 mmol/L. Serum magnesium concentrations were significantly lower in female than in male (0.73 Æ 0.12 vs 0.80 Æ 0.13 mmol/L), whereas the sex ratio M/F was higher in severe and critical form (p<0.001). In a bivariate analysis, the risk of magnesium deficiency was significantly and negatively associated with infection severity (p<0.001), sex ratio (M/F, p<0.001), oxygenotherapy (p<0.001), stay in critical care unit (p=0.028), and positively with nephropathy (p=0.026). -

Symptom Management for Cardiac Anorexia/Cachexia
Hospice Palliative Care HPC Consultation Services Waterloo Wellington Tip of the Month [email protected] May 2019 Palliative Approach to Care Tips: Symptom Management for Cardiac Anorexia/Cachexia • Focus interventions on treatment of Anorexia symptoms and reduction of psychological and social burden for patient and family. Anorexia is a syndrome characterized by loss of appetite, nausea, early satiety, weakness, fatigue, food aversion, and • Cachexia is not starvation. In cachexia, significant physical and/or psychological symptoms. Causes are complex and can include fatigue, dyspnea, medication side- effects, nausea, depression, anxiety and sodium-restricted diets, which are common to patients with heart failure. catabolism and subsequent weight loss continue to occur, even if caloric intake is Cachexia maintained or increased. Cachexia is a syndrome characterized by severe body weight, fat and muscle loss and increased protein catabolism due to • Educate patient/family about the underlying disease. This occurs in both chronic right-sided heart failure and the advanced stages of heart failure. difference between weight loss related to How is cachexia diagnosed? The patient with cachexia has: cachexia versus diuresis. • >5% non-edematous weight loss in <12 months; or body mass index (BMI) <20kg/m2; and • Artificial nutrition in the context of • 3 out of 5 of the following: fatigue, decreased muscle strength, anorexia, low muscle mass, abnormal biochemistry advanced cachexia is ineffective and will Screening not improve quality of life. • Screen with Edmonton Symptom Assessment System (ESAS-r) for issues with appetite, nausea, fatigue, depression. Assessment Figure 1: Understanding the Effects • of the Cachexia Cycle Obtain a thorough history of nutritional intake, weight loss, and symptoms (nausea, early satiety, dyspnea, poor oral hygiene, dysphagia, malabsorption, bowel habits). -

Eating Well to Prevent Vitamin B12 Deficiency
www.healthinfo.org.nz Eating well to prevent vitamin B12 deficiency Vitamin B12 helps keep your body's nerve and blood cells healthy. It helps make DNA, the genetic material in your cells. It also helps prevent a type of anaemia that can make you feel tired and weak. Causes of vitamin B12 deficiency Normally, your stomach and intestines digest and absorb vitamin B12 from your food. Vitamin B12 deficiency happens when your stomach and intestines can't absorb the vitamin. This can happen if any of the following apply. ▪ You have pernicious anaemia. This is where your body destroys the cells in your stomach that help you absorb vitamin B12. ▪ You have had surgery to remove part of your stomach or the last part of your small intestine. ▪ You have a digestive disorder such as coeliac disease or Crohn's disease. ▪ You are on certain long-term medications that make it harder for your body to absorb vitamin B12. These medications include antacids, heartburn medicines such as omeprazole and pantoprazole, and metformin. ▪ You are 65 or older. Vitamin B12 deficiency can also happen if you don't eat enough foods with vitamin B12. Most people in New Zealand get plenty of vitamin B12 from food. But some people might not get enough. These people include: ▪ vegans or strict vegetarians ▪ babies who are breastfed by mothers who are vegan or strict vegetarians ▪ people who eat little or no animal foods ▪ older people who have a poor appetite and eat very small meals. Treating vitamin B12 deficiency Vitamin B12 deficiency is diagnosed through a blood test. -

Is There an Ideal Diet to Protect Against Iodine Deficiency?
nutrients Review Is There an Ideal Diet to Protect against Iodine Deficiency? Iwona Krela-Ka´zmierczak 1,† , Agata Czarnywojtek 2,3,†, Kinga Skoracka 1,* , Anna Maria Rychter 1 , Alicja Ewa Ratajczak 1 , Aleksandra Szymczak-Tomczak 1, Marek Ruchała 2 and Agnieszka Dobrowolska 1 1 Department of Gastroenterology, Dietetics and Internal Diseases, Poznan University of Medical Sciences, Heliodor Swiecicki Hospital, 60-355 Poznan, Poland; [email protected] (I.K.-K.); [email protected] (A.M.R.); [email protected] (A.E.R.); [email protected] (A.S.-T.); [email protected] (A.D.) 2 Department of Endocrinology, Metabolism and Internal Medicine, Poznan University of Medical Sciences, 60-355 Poznan, Poland; [email protected] (A.C.); [email protected] (M.R.) 3 Department of Pharmacology, Poznan University of Medical Sciences, 60-806 Poznan, Poland * Correspondence: [email protected]; Tel.: +48-665-557-356 or +48-8691-343; Fax: +48-8691-686 † These authors contributed equally to this work. Abstract: Iodine deficiency is a global issue and affects around 2 billion people worldwide, with preg- nant women as a high-risk group. Iodine-deficiency prevention began in the 20th century and started with global salt iodination programmes, which aimed to improve the iodine intake status globally. Although it resulted in the effective eradication of the endemic goitre, it seems that salt iodination did not resolve all the issues. Currently, it is recommended to limit the consumption of salt, which is the main source of iodine, as a preventive measure of non-communicable diseases, such as hypertension or cancer the prevalence of which is increasing. -

Cachexia: the Physical and Psychosocial Impact
Cachexia: The physical and psychosocial impact Caroline Quilty RD, MSc Specialist Palliative Care Dietitian/Therapies Services Manager Aims What is cachexia & why should we be concerned about it? Consider the impact of anorexia in cachexia What are the physical and psychosocial effects of cachexia Identify our role in the management of patients with cachexia What is Cachexia? Cachexia in any disease refers to a state of severely and pathologically low weight, due principally to the loss of mass of tissues other than fat • a serious and under-recognised consequence of cancer (von Haeling and Ankers 2010) • Cachexia is a hallmark of certain diseases including cancer and COPD (Wagner 2008) What is cachexia? “a multifactorial syndrome characterised by an ongoing loss of skeletal muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutrition support, and progressive functional impairment. The pathophysiology is characterised by a negative protein and energy balance driven by a variable combination of reduced food intake and abnormal metabolism.” Fearon et al 2011 Prevalence Prevalence of cancer cachexia is high, ranging from 50-80% in advanced cancer (von Haeling and Ankers 2014) 20 – 40% of people with COPD have cachexia (von Haeling 2010) What does cachexia look like? The word cachexia has Greek roots, “kakos” meaning bad and “hexus” meaning habit, appearance, condition. Cachexia has been known for centuries (von Haehling 2010) and was described by Hippocrates “…the shoulders, clavicles, chest and thighs melt away” (Katz and Katz 1962). Some terms: Term Definition Ref Anorexia loss of appetite and diminished intake. Poole and Prevalent in cachexia Frogatt 2002 Malnutrition A state of nutrition in which a deficiency of energy, Elia 2003 protein and/or other nutrients causes measurable adverse effects on tissue/body form, composition, function or clinical outcome. -

Nutrition 102 – Class 3
Nutrition 102 – Class 3 Angel Woolever, RD, CD 1 Nutrition 102 “Introduction to Human Nutrition” second edition Edited by Michael J. Gibney, Susan A. Lanham-New, Aedin Cassidy, and Hester H. Vorster May be purchased online but is not required for the class. 2 Technical Difficulties Contact: Erin Deichman 574.753.1706 [email protected] 3 Questions You may raise your hand and type your question. All questions will be answered at the end of the webinar to save time. 4 Review from Last Week Vitamins E, K, and C What it is Source Function Requirement Absorption Deficiency Toxicity Non-essential compounds Bioflavonoids: Carnitine, Choline, Inositol, Taurine, and Ubiquinone Phytoceuticals 5 Priorities for Today’s Session B Vitamins What they are Source Function Requirement Absorption Deficiency Toxicity 6 7 What Is Vitamin B1 First B Vitamin to be discovered 8 Vitamin B1 Sources Pork – rich source Potatoes Whole-grain cereals Meat Fish 9 Functions of Vitamin B1 Converts carbohydrates into glucose for energy metabolism Strengthens immune system Improves body’s ability to withstand stressful conditions 10 Thiamine Requirements Groups: RDA (mg/day): Infants 0.4 Children 0.7-1.2 Males 1.5 Females 1 Pregnancy 2 Lactation 2 11 Thiamine Absorption Absorbed in the duodenum and proximal jejunum Alcoholics are especially susceptible to thiamine deficiency Excreted in urine, diuresis, and sweat Little storage of thiamine in the body 12 Barriers to Thiamine Absorption Lost into cooking water Unstable to light Exposure to sunlight Destroyed -

Biotinidase Deficiency: a Survey of 10 Cases
Arch Dis Child: first published as 10.1136/adc.63.10.1244 on 1 October 1988. Downloaded from Archives of Disease in Childhood, 1988, 63, 1244-1249 Biotinidase deficiency: a survey of 10 cases H J WASTELL,* K BARTLET,t G DALE,* AND A SHEIN *Department of Clinical Biochemistry, Newcastle General Hospital, and tDepartments of Child Health and Clinical Biochemistry, Newcastle University Medical School, Newcastle upon Tyne SUMMARY Ten patients with biotinidase deficiency were studied. Clinical findings at presenta- tion varied with dermatological signs (dermatitis and alopecia), neurological abnormalities (fits, hypotonia, and ataxia), and recurrent infections being the most common features, although none of these occurred in every case. Biochemically the disease is characterised by metabolic acidosis and organic aciduria. Treatment with biotin results in pronounced, rapid, clinical and biochemical improvement, but some patients have residual neurological damage comprising neurosensory hearing loss, visual pathway defects, ataxia, and mental retardation. The cause of this permanent damage remains obscure and it is not clear if the early introduction of treatment will prevent it. Biotin is a cofactor required by acetyl CoA carboxy- a functional biotin deficiency (biotinidase deficiency) lase (ACC) [EC 6.4.1.2], pyruvate carboxylase (PC) caused by failure to recycle endogenous biotin and [EC 6.4.1.1], propionyl CoA carboxylase (PCC) to liberate dietary biotin, or by defective biotinylation copyright. [EC 6.4.1.3] and 3 methylcrotonyl CoA carboxylase of apocarboxylase because of a mutant holocarboxy- (MCC) [EC 6.4.1.4.].' It is covalently attached to lase synthetase that has an increased Km with the apocarboxylases by the epsilon amino group of a respect to biotin. -

DRIDIETARY REFERENCE INTAKES Thiamin, Riboflavin, Niacin, Vitamin
Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline http://www.nap.edu/catalog/6015.html DIETARY REFERENCE INTAKES DRI FOR Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline A Report of the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline and Subcommittee on Upper Reference Levels of Nutrients Food and Nutrition Board Institute of Medicine NATIONAL ACADEMY PRESS Washington, D.C. Copyright © National Academy of Sciences. All rights reserved. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline http://www.nap.edu/catalog/6015.html NATIONAL ACADEMY PRESS • 2101 Constitution Avenue, N.W. • Washington, DC 20418 NOTICE: The project that is the subject of this report was approved by the Governing Board of the National Research Council, whose members are drawn from the councils of the National Academy of Sciences, the National Academy of Engineering, and the Institute of Medicine. The members of the committee responsible for the report were chosen for their special competences and with regard for appropriate balance. This project was funded by the U.S. Department of Health and Human Services Office of Disease Prevention and Health Promotion, Contract No. 282-96-0033, T01; the National Institutes of Health Office of Nutrition Supplements, Contract No. N01-OD-4-2139, T024, the Centers for Disease Control and Prevention, National Center for Chronic Disease Preven- tion and Health Promotion, Division of Nutrition and Physical Activity; Health Canada; the Institute of Medicine; and the Dietary Reference Intakes Corporate Donors’ Fund. -

Biotin Fact Sheet for Consumers
Biotin Fact Sheet for Consumers What is biotin and what does it do? Biotin is a B-vitamin found in many foods. Biotin helps turn the carbohydrates, fats, and proteins in the food you eat into the energy you need. How much biotin do I need? The amount of biotin you need each day depends on your age. Average daily recommended amounts are listed below in micrograms (mcg). Life Stage Recommended Amount Birth to 6 months 5 mcg Infants 7–12 months 6 mcg Children 1–3 years 8 mcg Children 4–8 years 12 mcg Biotin is naturally present in some Children 9–13 years 20 mcg foods, such as salmon and eggs. Teens 14–18 years 25 mcg Adults 19+ years 30 mcg Pregnant teens and women 30 mcg Breastfeeding teens and women 35 mcg What foods provide biotin? Many foods contain some biotin. You can get recommended amounts of biotin by eating a variety of foods, including the following: • Meat, fish, eggs, and organ meats (such as liver) • Seeds and nuts • Certain vegetables (such as sweet potatoes, spinach, and broccoli) What kinds of biotin dietary supplements are available? Biotin is found in some multivitamin/multimineral supplements, in B-complex supplements, and in supplements containing only biotin. Am I getting enough biotin? Most people get enough biotin from the foods they eat. However, certain groups of people are more likely than others to have trouble getting enough biotin: • People with a rare genetic disorder called “biotinidase deficiency” • People with alcohol dependence • Pregnant and breastfeeding women 2 • BIOTIN FACT SHEET FOR CONSUMERS What happens if I don’t get enough biotin? Biotin and healthful eating Biotin deficiency is very rare in the United States. -
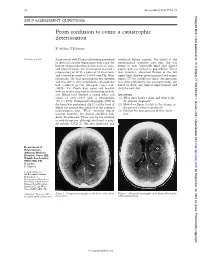
From Confusion to Coma: a Catastrophic Deterioration
52 Postgrad Med J 2001;77:52–55 Postgrad Med J: first published as 10.1136/pmj.77.903.53a on 1 January 2001. Downloaded from SELF ASSESSMENT QUESTIONS From confusion to coma: a catastrophic deterioration K Ashkan, F Johnston Answers on p 56. A previously well 45 year old woman presented ventilated before transfer. On arrival at the to the local casualty department with a one day neurosurgical intensive care unit, she was history of generalised headache, neck stiVness, found to have bilaterally fixed and dilated and blurred vision. On examination she had a pupils, with no corneal or gag reflexes. There temperature of 38°C, a pulse of 80 beats/min, was, however, abnormal flexion of the left and a blood pressure of 130/80 mm Hg. Neu- upper limb. She was given mannitol and urgent rologically, she had spontaneous eye opening repeat CT was carried out (fig 2). An operation and was able to obey commands, although she was then performed, but postoperatively she had confused speech (Glasgow coma scale failed to show any clinical improvement and (GCS), 14). Pupils were equal and reactive died the next day. with no cranial or peripheral neurological defi- cits. Blood tests showed a raised white cell Questions count of 20.9 × 109/l with a neutrophilia (1) What does figure 1 show and what is the (19.2 × 109/l). Computed tomography (CT) of diVerential diagnosis? the brain was performed (fig 1), on the basis of (2) How does figure 2 relate to the change in which the patient was referred to the regional the patient’s clinical condition? neurosurgical unit. -

Syllabus METAL ION EXCESS TOXICITY
Syllabus Metalionexcesstoxicity-Feexcesstoxicity-Africansiderosis,hemosiderosis, hemochromatosis(bronzediabetes)anddetoxification.Cuexcesstoxicity:Wilson’sdisease andtreatment. Heavymetaliontoxicity:Hg,Pb,Cd,Astoxiceffects–mechanismoftoxiceffects.Heavy metaltoxicitytreatment-chelationtherapy:chelatingagentsforHg,Pb,Cd,Astoxicity. Metalcomplexesasdrugs:cis-plainasanticanceragent:mechanismofactionandside effects;goldcomplexesasantiarthriticdrugs-chrysotherapy.Metalcomplexesindiagnosis- Gdcomplexesinmagneticresonanceimaging(MRI). METALIONEXCESSTOXICITY Ironexcesstoxicity ü ItisduetoaccidentalintakeofFeSO4tabletscausingerosionofthegastrointestinal tract. ü Hemochomatosisisageneticdisorder,depositionofironoccursinvitalorganslike liver,spleen,pancreas,skinetc.Itleadstobronzepigmentationontheskiniscalled bronzediabetes. ü Siderosis(depositionofFeOdustinthelungs)isassociatedwithexcessofiron. ü AfricansiderosisisanironoverloaddisorderfirstobservedamongpeopleofSouthern AfricaandCentralAfrica.Dietaryironoverloadistheconsumptionoflargeamount ofhome-brewedbeerwithhighamountofironcontentinit. Detoxification : Siderophore desferrioxamine is a chelating antidote used for Fe removal Coppertoxicity Copper is a principal component of several metalloproteins and some naturally occurring pigments.Ahealthyadultpossessescopperbetween200to300mgandthehighestamountis concentratedin the locus of brain. Wilson’sdiseaseand Menkes’ kinky hair syndromeare associatedwithageneticdisorderinthemetabolismofcopper. Wilson’sdisease ü In Wilson'sdisease,the copper-content