Principles and Methods for the Assessment of Neurotoxicity Associated with Exposure to Chemicals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Toxicology Mechanisms Underlying the Neurotoxicity Induced by Glyphosate-Based Herbicide in Immature Rat Hippocampus
Toxicology 320 (2014) 34–45 Contents lists available at ScienceDirect Toxicology j ournal homepage: www.elsevier.com/locate/toxicol Mechanisms underlying the neurotoxicity induced by glyphosate-based herbicide in immature rat hippocampus: Involvement of glutamate excitotoxicity Daiane Cattani, Vera Lúcia de Liz Oliveira Cavalli, Carla Elise Heinz Rieg, Juliana Tonietto Domingues, Tharine Dal-Cim, Carla Inês Tasca, ∗ Fátima Regina Mena Barreto Silva, Ariane Zamoner Departamento de Bioquímica, Centro de Ciências Biológicas, Universidade Federal de Santa Catarina, Florianópolis, Santa Catarina, Brazil a r a t i b s c l t r e a i n f o c t Article history: Previous studies demonstrate that glyphosate exposure is associated with oxidative damage and neu- Received 23 August 2013 rotoxicity. Therefore, the mechanism of glyphosate-induced neurotoxic effects needs to be determined. Received in revised form 12 February 2014 ® The aim of this study was to investigate whether Roundup (a glyphosate-based herbicide) leads to Accepted 6 March 2014 neurotoxicity in hippocampus of immature rats following acute (30 min) and chronic (pregnancy and Available online 15 March 2014 lactation) pesticide exposure. Maternal exposure to pesticide was undertaken by treating dams orally ® with 1% Roundup (0.38% glyphosate) during pregnancy and lactation (till 15-day-old). Hippocampal Keywords: ® slices from 15 day old rats were acutely exposed to Roundup (0.00005–0.1%) during 30 min and experi- Glyphosate 45 2+ Calcium ments were carried out to determine whether glyphosate affects Ca influx and cell viability. Moreover, 14 we investigated the pesticide effects on oxidative stress parameters, C-␣-methyl-amino-isobutyric acid Glutamatergic excitotoxicity 14 Oxidative stress ( C-MeAIB) accumulation, as well as glutamate uptake, release and metabolism. -

Crews Lab Discovers Inflammatory Mechanisms Underlying Alcohol
research alcohol actions on brain, liver, pancreas, fetus, the The Director’s Column endocrine system and the immune system, has contributed Thanks for your gifts to our Center! to Crews’ insights. Alternatively, I suspect that he A. Leslie Morrow, Ph.D. assembled this diverse group of researchers to study alcohol Dean’’’ s Club Ms. Carolyn S. Corn Mr. and Mrs. Ben Madore Associate Director, actions across so many systems of the body for the very Ms. Harriet W. Crisp Bowles Center for (annual gifts of $5000 and above) Ms. Regina J. Mahon purpose of discovering how the complex systemic and Mr. and Mrs. Ray Damerell Alcohol Studies Dr. William Maixner molecular roles of alcohol go awry in the disease of Center Line James A. Bowles Mr. Lewis A. Dancy Mrs. Melissa M. Mann Bowles Center for Alcohol Studies alcoholism. Mr. John N. Howard, Jr. Mr. F. E. Danielus Mr. and Mrs. H. J. McCarthy School of Medicine, University of North Carolina at Chapel Hill Mr. and Mrs. Robert F. Schaecher Mr. Stephen M. Dean and Ms. Patricia L. Ms. Michelle McCarthy This integration of research across systems, organs, brain Ms. Ann Lewallen Spencer Amend Mr. and Mrs. Robert H. McConville, Jr. Dr. Fulton Crews’ brilliance can be attributed in part to regions, human development and the progression to Our mission is to conduct, coordinate, and promote basic and clinical research on the causes, prevention, and treatment of alcoholism and alcoholic disease. Tennessee Medical Foundation Ms. Elizabeth L. Deaver Mr. and Mrs. Loyce L. McCormick his understanding that human beings are not just a alcoholism is the hallmark of research in the Bowles Center Ms. -

Assessing Neurotoxicity of Drugs of Abuse
National Institute on Drug Abuse RESEARCH MONOGRAPH SERIES Assessing Neurotoxicity of Drugs of Abuse 136 U.S. Department of Health and Human Services • Public Health Service • National Institutes of Health Assessing Neurotoxicity of Drugs of Abuse Editor: Lynda Erinoff, Ph.D. NIDA Research Monograph 136 1993 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service National Institutes of Health National Institute on Drug Abuse 5600 Fishers Lane Rockville, MD 20857 ACKNOWLEDGMENT This monograph is based on the papers and discussions from a technical review on “Assessing Neurotoxicity of Drugs of Abuse” held on May 20-21, 1991, in Bethesda, MD. The technical review was sponsored by the National Institute on Drug Abuse (NIDA). COPYRIGHT STATUS NIDA has obtained permission from the copyright holders to reproduce certain previously published material as noted in the text. Further reproduction of this copyrighted material is permitted only as part of a reprinting of the entire publication or chapter. For any other use, the copyright holder’s permission is required. All other material in this volume except quoted passages from copyrighted sources is in the public domain and may be used or reproduced without permission from the Institute or the authors. Citation of the source is appreciated. Opinions expressed in this volume are those of the authors and do not necessarily reflect the opinions or official policy of the National Institute on Drug Abuse or any other part of the U.S. Department of Health and Human Services. The U.S. Government does not endorse or favor any specific commercial product or company. -

Suspect and Target Screening of Natural Toxins in the Ter River Catchment Area in NE Spain and Prioritisation by Their Toxicity
toxins Article Suspect and Target Screening of Natural Toxins in the Ter River Catchment Area in NE Spain and Prioritisation by Their Toxicity Massimo Picardo 1 , Oscar Núñez 2,3 and Marinella Farré 1,* 1 Department of Environmental Chemistry, IDAEA-CSIC, 08034 Barcelona, Spain; [email protected] 2 Department of Chemical Engineering and Analytical Chemistry, University of Barcelona, 08034 Barcelona, Spain; [email protected] 3 Serra Húnter Professor, Generalitat de Catalunya, 08034 Barcelona, Spain * Correspondence: [email protected] Received: 5 October 2020; Accepted: 26 November 2020; Published: 28 November 2020 Abstract: This study presents the application of a suspect screening approach to screen a wide range of natural toxins, including mycotoxins, bacterial toxins, and plant toxins, in surface waters. The method is based on a generic solid-phase extraction procedure, using three sorbent phases in two cartridges that are connected in series, hence covering a wide range of polarities, followed by liquid chromatography coupled to high-resolution mass spectrometry. The acquisition was performed in the full-scan and data-dependent modes while working under positive and negative ionisation conditions. This method was applied in order to assess the natural toxins in the Ter River water reservoirs, which are used to produce drinking water for Barcelona city (Spain). The study was carried out during a period of seven months, covering the expected prior, during, and post-peak blooming periods of the natural toxins. Fifty-three (53) compounds were tentatively identified, and nine of these were confirmed and quantified. Phytotoxins were identified as the most frequent group of natural toxins in the water, particularly the alkaloids group. -

The Neurotoxin Β-N-Methylamino-L-Alanine (BMAA)
The neurotoxin β-N-methylamino-L-alanine (BMAA) Sources, bioaccumulation and extraction procedures Sandra Ferreira Lage ©Sandra Ferreira Lage, Stockholm University 2016 Cover image: Cyanobacteria, diatoms and dinoflagellates microscopic pictures taken by Sandra Ferreira Lage ISBN 978-91-7649-455-4 Printed in Sweden by Holmbergs, Malmö 2016 Distributor: Department of Ecology, Environment and Plant Sciences, Stockholm University “Sinto mais longe o passado, sinto a saudade mais perto.” Fernando Pessoa, 1914. Abstract β-methylamino-L-alanine (BMAA) is a neurotoxin linked to neurodegeneration, which is manifested in the devastating human diseases amyotrophic lateral sclerosis, Alzheimer’s and Parkinson’s disease. This neurotoxin is known to be produced by almost all tested species within the cyanobacterial phylum including free living as well as the symbiotic strains. The global distribution of the BMAA producers ranges from a terrestrial ecosystem on the Island of Guam in the Pacific Ocean to an aquatic ecosystem in Northern Europe, the Baltic Sea, where annually massive surface blooms occur. BMAA had been shown to accumulate in the Baltic Sea food web, with highest levels in the bottom dwelling fish-species as well as in mollusks. One of the aims of this thesis was to test the bottom-dwelling bioaccumulation hy- pothesis by using a larger number of samples allowing a statistical evaluation. Hence, a large set of fish individuals from the lake Finjasjön, were caught and the BMAA concentrations in different tissues were related to the season of catching, fish gender, total weight and species. The results reveal that fish total weight and fish species were positively correlated with BMAA concentration in the fish brain. -

Clinical Neurophysiology Board Review Q&A
Clinical Neurophysiology Board Review Board Clinical Neurophysiology Clinical Neurophysiology Board Review Q&A Clinical Puneet K. Gupta, MD, MSE • Pradeep N. Modur, MD, MS • Srikanth Muppidi, MD his high-yield, illustrated clinical neurophysiology board review is a comprehen- Neurophysiology sive resource for assessing and refining the knowledge tested on multiple board Texaminations. Written by authors who are collectively board certified in all of the areas covered, the book is a valuable study tool for candidates preparing for certifica- tion or recertification in clinical neurophysiology, neuromuscular medicine, epilepsy, Board Review sleep medicine, and neurology. Using structured question formats typically encountered on boards, this comprehensive review allows users to assess their knowledge in a wide range of topics, provides rationales for correct answers, and explains why the other choices are incorrect. A unique “Pearls” section at the end of the book allows for quick review of the most important concepts prior to exam day. Clinical Neurophysiology Board Review Q&A contains 801 questions with answers and detailed explanations. The book is divided into eight chapters covering anatomy Q and physiology, electronics and instrumentation, nerve conduction studies and EMG, & EEG, evoked potentials and intraoperative monitoring, sleep studies, ethics and safety, and advanced topics including QEEG, MEG, TES, autonomic testing, and more. A Liberal use of image-based questions illustrating the full spectrum of neurophysiologic & tests and findings build interpretive skills. Questions are randomized and include Q A both case-related questions in series and stand-alone items to familiarize candidates Gu with the question types and formats they will find on the exam. -
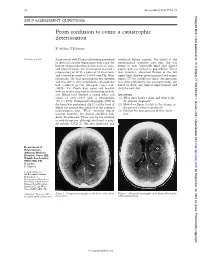
From Confusion to Coma: a Catastrophic Deterioration
52 Postgrad Med J 2001;77:52–55 Postgrad Med J: first published as 10.1136/pmj.77.903.53a on 1 January 2001. Downloaded from SELF ASSESSMENT QUESTIONS From confusion to coma: a catastrophic deterioration K Ashkan, F Johnston Answers on p 56. A previously well 45 year old woman presented ventilated before transfer. On arrival at the to the local casualty department with a one day neurosurgical intensive care unit, she was history of generalised headache, neck stiVness, found to have bilaterally fixed and dilated and blurred vision. On examination she had a pupils, with no corneal or gag reflexes. There temperature of 38°C, a pulse of 80 beats/min, was, however, abnormal flexion of the left and a blood pressure of 130/80 mm Hg. Neu- upper limb. She was given mannitol and urgent rologically, she had spontaneous eye opening repeat CT was carried out (fig 2). An operation and was able to obey commands, although she was then performed, but postoperatively she had confused speech (Glasgow coma scale failed to show any clinical improvement and (GCS), 14). Pupils were equal and reactive died the next day. with no cranial or peripheral neurological defi- cits. Blood tests showed a raised white cell Questions count of 20.9 × 109/l with a neutrophilia (1) What does figure 1 show and what is the (19.2 × 109/l). Computed tomography (CT) of diVerential diagnosis? the brain was performed (fig 1), on the basis of (2) How does figure 2 relate to the change in which the patient was referred to the regional the patient’s clinical condition? neurosurgical unit. -

The Peripheral Nerves: Update on Ultrasound and Magnetic Resonance Imaging I
The peripheral nerves: update on ultrasound and magnetic resonance imaging I. Möller1, M. Miguel2, D.A. Bong1, F. Zaottini3, C. Martinoli4 1Instituto Poal de Reumatologia, ABSTRACT of the nerve along its trajectory along University of Barcelona, Spain, The motor and sensory branches of with immediate one-to-one compari- and EULAR Working Group Anatomy the somatic peripheral nervous system son with the contralateral structures (6, for the Image; (PNS) can be visualised by different im- 7). In addition, US-guidance has led to 2Department of Pathology and Experimental Therapeutics, Human aging systems. This article focuses on the development of a variety of inter- Anatomy and Embryology Unit, imaging of peripheral nerves by mag- ventional procedures. The use of US is University of Barcelona, Spain; netic resonance imaging (MRI) and becoming widespread in providing ac- 3Department of Health Sciences, high-resolution ultrasound (US). The curate and safe regional anesthesia as DISSAL, University of Genoa; anatomic basis of the peripheral nerve well as focal and regional pain manage- 4 Department of Health Science, image, common pathologies and clini- ment. It has also becoming an increas- University of Genoa, Ospedale cal value of US and MRI imaging of pe- ingly important component of muscu- Policlinico San Martino, Genoa, Italy. ripheral nerves are reviewed. loskeletal specialties such as physical Ingrid Möller, MD medicine and rehabilitation and sports Maribel Miguel, MD David A. Bong, MD Introduction medicine. Federico Zaottini, MD Nerve pathology may be a cause of Carlo Martinoli, MD chronic pain and disability. The initial Anatomical considerations Please address correspondence to: diagnostic evaluation of the periph- The PNS includes spinal nerves that Dr David A. -

Hypervitaminosis - an Emerging Pathological Condition
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Review Article Hypervitaminosis - An Emerging Pathological Condition J K Roop PG Department of Zoology, JC DAV College (Affiliated to Panjab University, Chandigarh), Dasuya-144205, District Hoshiarpur, Punjab, India ABSTRACT Vitamins are essential organic compounds that are required in small amounts to regulate various metabolic activities in the body. Prolonged and overconsumption of pharmaceutical forms of both water-soluble and fat-soluble vitamins may lead to toxicity and/or hypervitaminosis. Hypervitaminosis is an acute emerging pathological condition of the body due to excess accumulation of any of the vitamins. In case of acute poisoning with vitamin supplements/drugs, emergency assistance is required to detoxify the effects and restore the organization, structure and function of body’s tissues and organs. Sometimes death may occur due to intoxication to liver, kidney and heart. So, to manage any type of hypervitaminosis, proper diagnosis is essential to initiate eliminating the cause of its occurrence and accelerate the elimination of the supplement from the body. The present review discusses the symptoms of hypervitaminosis that seems to be a matter of concern today and management strategies to overcome toxicity or hypervitaminosis. Keywords: Hypervitaminosis, Toxicity, Vitamins, Vitamin pathology, Fat-soluble vitamin, Water- soluble vitamin. INTRODUCTION body, particularly in the liver. Vitamin B Vitamins are potent organic Complex and vitamin C are water- soluble. compounds present in small concentrations They are dissolved easily in food during in various fruits and vegetables. They cooking and a portion of these vitamins may regulate physiological functions and help in be destroyed by heating. -

The Practice and Regulatory Requirements Of
School of Public Health The Practice and Regulatory Requirements of Naturopathy and Western Herbal Medicine Vivian Lin Alan Bensoussan Stephen P. Myers Pauline McCabe Marc Cohen Sophie Hill Genevieve Howse November, 2005 Funded by the Department of Human Services, Victoria Copyright State of Victoria, Department of Human Services, 2006 This publication is copyright. No part may be reproduced by any process except in accordance with the provisions of the Copyright Act 1968 (Cth). Authorised by the State Government of Victoria, 50 Lonsdale Street Melbourne. This document may be downloaded from the following website: www.health.vic.gov.au/pracreg/naturopathy.htm ISBN-13: 978-0-9775864-0-0 ISBN-10: 0-9775864-0-5 Published by School of Public Health, La Trobe University Bundoora Victoria, 3086 Australia THE PRACTICE AND REGULATORY REQUIREMENTS OF NATUROPATHY AND WESTERN HERBAL MEDICINE CONTENTS VOLUME ONE Summary Report..........................................................................................................1 1. Introduction.......................................................................................................1 2. Methodology.....................................................................................................1 3. Key findings and recommendations..................................................................2 3.1 Definition of practice and scope of study ........................................................2 3.2 Growing use of naturopathy and WHM...........................................................3 -

EMG (Electromyography) And/Or NCS (Nerve Conduction Studies)
Tempe ñ Phoenix ñ Gilbert ñ Scottsdale ñ Peoria ñ Show Low PHONE: (480) 962-0071 www.SonoranSpine.com ñ www.SpineResearch.org Patient Name: Date: Date of Birth: S O N O R A N S P I N E -- R E F E R E N C E Electromyography and Nerve Conduction Studies An electromyogram (EMG) measures the electrical The electrode will be moved a number of times to activity of muscles at rest and during contraction. record the activity in different areas of the muscle or in Nerve conduction studies (NCS) measure how well and different muscles. how fast the nerves can send electrical signals. Nerve conduction studies If you have leg pain or numbness, you may undergo Several electrodes are attached to your skin. Several these tests to determine how much your nerves are quick electrical pulses are given to the nerve, and the being affected. These tests check how well the spinal time it takes for the muscle to contract in response to nerves and the nerves in your arms and legs are the electrical pulse is recorded. The speed of the working and whether there is nerve irritation or response is called the conduction velocity. The same damage. They do not test for pain. nerves on the other side of the body may be studied for comparison. When the test is done, the electrodes Why It Is Done are removed. An EMG is done to determine muscle tissue or nerve damage. It can find the cause of weakness, paralysis, How It Feels or muscle twitching. -

Hepatic Pathology in Vitamin a Toxicity FAIRPOUR FOROUHAR, M.D.* MARTIN S
ANNALS OF CLINICAL AND LABORATORY SCIENCE, Vol. 14, No. 4 Copyright © 1984, Institute for Clinical Science, Inc. Hepatic Pathology in Vitamin A Toxicity FAIRPOUR FOROUHAR, M.D.* MARTIN S. NADEL, M.D.,t and BERNARD GONDOS, M.D.* *Department of Pathology, University of Connecticut, Farmington, CT 06032 and fDepartment of Pathology, Middlesex Memorial Hospital, Middletown, CT 06457 ABSTRACT This paper documents a case of vitamin A toxicity presenting with splenomegaly and ascites. The light microscopic, electron microscopic, and fluorescent findings are described in detail. The principal histopath ologic finding was marked perisinusoidal fibrosis. The role of Ito cells in the storage of lipid-soluble vitamins and their subsequent transformation to fibroblasts producing collagen are discussed. tient denied use of medications but admitted long Introduction time use of a self-prescribed vitamin B1-B6 combi nation, vitamin B12, iodine, and garlic. Vitamin A Liver disease resulting from vitamin A had been taken for six years, initially 75,000 units toxicity is an uncommon but well docu daily, increased to 150,000 units daily two years prior to admission because of believed problems with night mented entity.6’1414’22’26,27 The present vision for which no medical help had been sought. case provides a good model for the study Physical examination disclosed no palpable spleno of fibrosis of the space of Disse and its megaly with liver felt one cm beneath the right costal margin. An abdominal fluid wave was present. No pathophysiologic ^consequences. Similar spider angiomata were noted, and the remainder of changes also occur in other conditions re the physical examination was unremarkable.