Chapter 18 Ethers and Epoxides; Thiols and Sulfides Ethers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 21 the Chemistry of Carboxylic Acid Derivatives
Instructor Supplemental Solutions to Problems © 2010 Roberts and Company Publishers Chapter 21 The Chemistry of Carboxylic Acid Derivatives Solutions to In-Text Problems 21.1 (b) (d) (e) (h) 21.2 (a) butanenitrile (common: butyronitrile) (c) isopentyl 3-methylbutanoate (common: isoamyl isovalerate) The isoamyl group is the same as an isopentyl or 3-methylbutyl group: (d) N,N-dimethylbenzamide 21.3 The E and Z conformations of N-acetylproline: 21.5 As shown by the data above the problem, a carboxylic acid has a higher boiling point than an ester because it can both donate and accept hydrogen bonds within its liquid state; hydrogen bonding does not occur in the ester. Consequently, pentanoic acid (valeric acid) has a higher boiling point than methyl butanoate. Here are the actual data: INSTRUCTOR SUPPLEMENTAL SOLUTIONS TO PROBLEMS • CHAPTER 21 2 21.7 (a) The carbonyl absorption of the ester occurs at higher frequency, and only the carboxylic acid has the characteristic strong, broad O—H stretching absorption in 2400–3600 cm–1 region. (d) In N-methylpropanamide, the N-methyl group is a doublet at about d 3. N-Ethylacetamide has no doublet resonances. In N-methylpropanamide, the a-protons are a quartet near d 2.5. In N-ethylacetamide, the a- protons are a singlet at d 2. The NMR spectrum of N-methylpropanamide has no singlets. 21.9 (a) The first ester is more basic because its conjugate acid is stabilized not only by resonance interaction with the ester oxygen, but also by resonance interaction with the double bond; that is, the conjugate acid of the first ester has one more important resonance structure than the conjugate acid of the second. -

Organic Chemistry
Wisebridge Learning Systems Organic Chemistry Reaction Mechanisms Pocket-Book WLS www.wisebridgelearning.com © 2006 J S Wetzel LEARNING STRATEGIES CONTENTS ● The key to building intuition is to develop the habit ALKANES of asking how each particular mechanism reflects Thermal Cracking - Pyrolysis . 1 general principles. Look for the concepts behind Combustion . 1 the chemistry to make organic chemistry more co- Free Radical Halogenation. 2 herent and rewarding. ALKENES Electrophilic Addition of HX to Alkenes . 3 ● Acid Catalyzed Hydration of Alkenes . 4 Exothermic reactions tend to follow pathways Electrophilic Addition of Halogens to Alkenes . 5 where like charges can separate or where un- Halohydrin Formation . 6 like charges can come together. When reading Free Radical Addition of HX to Alkenes . 7 organic chemistry mechanisms, keep the elec- Catalytic Hydrogenation of Alkenes. 8 tronegativities of the elements and their valence Oxidation of Alkenes to Vicinal Diols. 9 electron configurations always in your mind. Try Oxidative Cleavage of Alkenes . 10 to nterpret electron movement in terms of energy Ozonolysis of Alkenes . 10 Allylic Halogenation . 11 to make the reactions easier to understand and Oxymercuration-Demercuration . 13 remember. Hydroboration of Alkenes . 14 ALKYNES ● For MCAT preparation, pay special attention to Electrophilic Addition of HX to Alkynes . 15 Hydration of Alkynes. 15 reactions where the product hinges on regio- Free Radical Addition of HX to Alkynes . 16 and stereo-selectivity and reactions involving Electrophilic Halogenation of Alkynes. 16 resonant intermediates, which are special favor- Hydroboration of Alkynes . 17 ites of the test-writers. Catalytic Hydrogenation of Alkynes. 17 Reduction of Alkynes with Alkali Metal/Ammonia . 18 Formation and Use of Acetylide Anion Nucleophiles . -

1.1 10 Oxidation of Alcohols and Aldehydes
1.1 10 Oxidation of alcohols and aldehydes By the end of this spread, you should be able to … 1Describe the oxidation of primary alcohols to form aldehydes and carboxylic acids. 1Describe the oxidation of secondary alcohols to form ketones. 1Describe the oxidation of aldehydes to form carboxylic acids. Key definition Oxidation of alcohols You will recall from your AS chemistry studies that primary and secondary alcohols can A redox reaction is one in which both reduction and oxidation take place. be oxidised using an oxidising agent. s !SUITABLEOXIDISINGAGENTISASOLUTIONCONTAININGACIDIlEDDICHROMATEIONS + 2− H /Cr2O7 . s 4HEOXIDISINGMIXTURECANBEMADEFROMPOTASSIUMDICHROMATE +2Cr2O7, and sulfuric acid, H2SO. During the reaction, the acidified potassium dichromate changes from orange to green. Remember that tertiary alcohols are not oxidised by acidified dichromate ions. Primary alcohols Primary alcohols can be oxidised to aldehydes and to carboxylic acids (see AS Chemistry spread 2.2.3). H H H O H O Oxidation Oxidation H CCOH H CC H CC H H H H H OH Primary alcohol Aldehyde Carboxylic acid Figure 1 Ethanol oxidised to ethanal, and finally to ethanoic acid The equation for the oxidation of ethanol to the aldehyde ethanal is shown below. Note that the oxidising agent is shown as [O] – this simplifies the equation. CH3CH2OH + [O] }m CH3CHO + H2O When preparing aldehydes in the laboratory, you will need to distil the aldehyde from the reaction mixture as it is formed. This prevents the aldehyde from being oxidised further to a carboxylic acid. Key definition When making the carboxylic acid, the reaction mixture is usually heated under reflux before distilling the product off. -

United States Patent 15) 3,669,956 Borck Et Al
United States Patent 15) 3,669,956 Borck et al. (45) June 13, 1972 54) 4-SUBSTITUTEDAMNO 260/472, 260/516, 260/518 R, 260/518 A, 260/519, PHENYACETIC ACDS AND 260/556 AR, 260/556 B, 260/558 S, 260/558 A, DERVATIVES THEREOF 260/559 T, 260/559 A, 260/.571, 260/574, 260/.575, (72 Inventors: Joachim Borck; Johann Dahin; Volker 424/244, 424/246, 424/248, 424/250, 424/267, Koppe; Josef Kramer; Gustav Shorre; J. 424/270, 424/272, 424/273, 424/274, 424/304, W. Hermann Hovy; Ernst Schorscher, all 424/309, 424/32 i, 424/324, 424/330 of Darmstadt, Germany 51) int. Cl. ........................................................ C07d 41/04 58) Field of Search........ 260/294X,293.4, 293.47, 239 BF, 73) Assignee: E. Merck A. G., Darmstadt, Germany 260/326.3, 294.3 E (22) Filed: July 22, 1968 56) References Cited (21) Appl. No.: 746,326 UNITED STATES PATENTS (30) Foreign Application Priority Data 3,252,970 5/1966 Huebner................................ 260/239 July 22, 1967 Germany.............................. M 74881 3,385,852 5/1968 Casadio................................. 260/246 Jan. 8, 1968 Germany... ....M 76850 OTHER PUBLICATIONS Feb. 23, 1968 Germany...... ...M 77363 March 1, 1968 Germany.............................. M 77429 Norman et al., J. Chen. Soc. 1963, (Nov.), 5431-6. (52) U.S. Cl................... 260/239 BF, 260/239 A, 260/239 E, Primary Examiner-Henry R. Jiles 260/243 B, 260/246, 260/247. 1, 260/247.2 R, Assistant Examiner-G. Thomas Todd 260/247.2 A, 260/247.2 B, 260/247.5 R, 260/247.7 Attorney-Millen, Raptes & White A, 260/247.7 H, -

Exam 1 (February 23, 2004) ID# ______
Chemistry 211 Name ___________________________ Exam 1 (February 23, 2004) ID# ___________________________ 10 1. You desire to synthesize 3-ethyl-3-pentanol starting with an ester. (i) What would be the name of the ester, and what is the name for the Grignard reagent (e.g., methyl magnesium bromide)? (ii) For the carbons shown in the product, show plausible hydrocarbons that you could start with to produce the ester and the Grignard reagent (as in a retrosynthesis). 12 2. (i) Show the step-by-step process required to produce propyllithium, which requires a free radical reaction mechanism, . (ii) Show the complete reaction mechanism for reaction between propyllithium and the correct ketone to produce 3-propyl-3-pentanol. (iii) Propose a possible reaction mechanism by which dipropyl cuprate (Cu+ with two propyl groups attached) could react with ethyl bromide to produce a new hydrocarbon. (This is a thinking exercise! So, think! () 8 3. As mentioned in the text, diethyl ether, pentane, and 1-butanol have similar molar masses, but different physical properties. Boiling points are 35oC, 36oC, and 117oC, respectively. Their respective solubilities in water are 7.5g/100mL, insoluble, and 9g/100mL. (i) Draw structures for each of these compounds. (ii) Justify the observed boiling points and their solubilities. 16 4. Draw structures of the following compounds 2,3-heptanediol isopropyllithium benzylmagnesium bromide benzoic acid benzaldehylde dimethyl sulfide t-butyl methanoate dibutyl ketone 12 5. Alcohols can be oxidized to produce other compounds, and can be produced by reduction. For the reactions shown below, show the structure for the expected product (if reaction does not occur, state: No Reaction) when treated with the indicated oxidizing or reducing agents. -

Functionalized Hybrid Silicones – Catalysis, Synthesis and Application
Technische Universität München Fakultät für Chemie Fachgebiet Molekulare Katalyse Functionalized Hybrid Silicones – Catalysis, Synthesis and Application Sophie Luise Miriam Putzien Vollständiger Abdruck der von der Fakultät für Chemie der Technischen Universität München zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften (Dr. rer. nat.) genehmigten Dissertation. Vorsitzender: Univ.-Prof. Dr. M. Schuster Prüfer der Dissertation: 1. Univ.-Prof. Dr. F. E. Kühn 2. Univ.-Prof. Dr. O.Nuyken (i.R.) Die Dissertation wurde am 16.02.2012 bei der Technischen Universität München eingereicht und durch die Fakultät für Chemie am 08.03.2012 angenommen. The following dissertation was prepared between April 2009 and March 2012 at the Chair of Inorganic Chemistry, Department of Molecular Catalysis of the Technische Universität München. I would like to express my deep gratitude to my academic supervisor Prof. Dr. Fritz E. Kühn for his support and confidence and the freedom of scientific research. This work was supported by a research grant from the BASF Construction Chemicals GmbH, Trostberg, Germany. Acknowledgement I would like to express my sincere gratitude to Prof. Dr. Oskar Nuyken and Dr. Eckhart Louis for their ongoing support and their undamped enthusiasm for my research topic. They supported this work with many inspiring discussions, new ideas and critical questions. I thank the BASF Construction Chemicals GmbH, Trostberg, for giving me the opportunity to work on an industrial cooperation project. Especially, I would like to thank Dr. Simone Klapdohr and Dr. Burkhard Walther, who accompanied this project from the industrial perspectice, for their support and the nice time I had in Trostberg during the application technological tests. -

Chemistry for S5 Students Short Notes & Questions with Answers & And
Chemistry for S5 students Short notes & questions with answers & and other questions to help S5 Students to revise Important Concepts: Chemical Reactions of Alkyl Halides The reaction can be broadly classified in two categories: (a) Nucleophilic substitution (b) Elimination reactions Nucleophilic substitution reactions: In this reaction a nucleophile, which is rich in electrons, attacks partial positive charge on the carbon atom bonded to halogen to replace the leaving group. Nucleophilic reactions proceed by two different mechanism: (a) Substitution nucleophilic bimolecular (SN2) (b) Substitution nucleophilic unirnolecular (SN1) The reaction follows second order kinetics No intermediate is formed. It usually requires a strong nucleophile. The order of reactivity followed as: Primary halide > Secondary halide > Tertiary halide It is carried out in polar protic solvents (water, Alcohol, acetic acid etc.). These reactions occur in two steps as shown above The order of leaving ability is: F- < Cl- < Br- < I- The order of reactivity is as shown below: Difference between E1 and E2 reaction mechanism: Attributes E1 E2 Rate law Depend on the concentration of Depends on the concentration of both substrate substrate and base Barrier Formation of carbocation None 3o>2o>> 1o Base Does not require strong base Requires strong base Stereochemistry Does not require stereochemistry Leaving group must be anti to hydrogen removed Some solved questions are given below: Question 1:Which is the correct increasing order of boiling points of the following compounds? 1-bromoethane, 1-bromopropane, 1-bromobutane, Bromobenzene (a) Bromobenzene < 1-bromobutane < 1-bromopropane < 1-bromoethane (b) Bromobenzene < 1-bromothane < 1-bromopropane < 1-bromobutane (c) 1-bromopropane < 1-bromobutane < 1-bromoethane < Bromobenzene (d) 1-bromoethane < 1-bromopropane < 1-bromobutane < Bromobenzene Solution 1: Boiling point increases with increase in size of hydrocarbon part for the same haloalkanes. -

Understanding the Thermochemical Conversion of Biomass to Overcome Biomass Recalcitrance Kwang Ho Kim Iowa State University
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2015 Understanding the thermochemical conversion of biomass to overcome biomass recalcitrance Kwang Ho Kim Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Agriculture Commons, and the Bioresource and Agricultural Engineering Commons Recommended Citation Kim, Kwang Ho, "Understanding the thermochemical conversion of biomass to overcome biomass recalcitrance" (2015). Graduate Theses and Dissertations. 14382. https://lib.dr.iastate.edu/etd/14382 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Understanding the thermochemical conversion of biomass to overcome biomass recalcitrance by Kwang Ho Kim A dissertation submitted to the graduate faculty in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Major: Agricultural and Biosystems Engineering Program of Study Committee: Robert C. Brown, Co-Major Professor Xianglan Bai, Co-Major Professor Kurt Rosentrater Matthew Darr Brent Shanks Young Jin Lee Iowa State University Ames, Iowa 2015 Copyright © Kwang Ho Kim, 2015. All rights reserved. ii DEDICATION Dedicated to my family for their unwavering support -
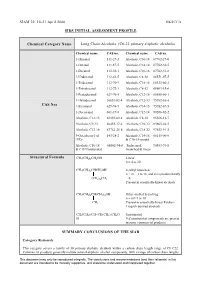
Long Chain Alcohols (C6-22 Primary Aliphatic Alcohols)
SIAM 22, 18-21 April 2006 UK/ICCA SIDS INITIAL ASSESSMENT PROFILE Chemical Category Name Long Chain Alcohols (C6-22 primary aliphatic alcohols) Chemical name CAS no. Chemical name CAS no. 1-Hexanol 111-27-3 Alcohols, C16-18 67762-27-0 1-Octanol 111-87-5 Alcohols, C14-18 67762-30-5 1-Decanol 112-30-1 Alcohols, C10-16 67762-41-8 1-Undecanol 112-42-5 Alcohols, C8-18 68551-07-5 1-Tridecanol 112-70-9 Alcohols, C14-16 68333-80-2 1-Tetradecanol 112-72-1 Alcohols, C6-12 68603-15-6 1-Pentadecanol 629-76-5 Alcohols, C12-16 68855-56-1 1-Hexadecanol 36653-82-4 Alcohols, C12-13 75782-86-4 CAS Nos 1-Eicosanol 629-96-9 Alcohols, C14-15 75782-87-5 1-Docosanol 661-19-8 Alcohols, C12-14 80206-82-2 Alcohols, C12-15 63393-82-8 Alcohols, C8-10 85566-12-7 Alcohols, C9-11 66455-17-2 Alcohols, C10-12 85665-26-5 Alcohols, C12-18 67762-25-8 Alcohols, C18-22 97552-91-5 9-Octadecen-1-ol 143-28-2 Alcohols, C14-18. 68155-00-0 (9Z)- & C16-18-unsatd Alcohols, C16-18 68002-94-8 Tridecanol, 90583-91-8 & C18 Unsaturated branched & linear Structural Formula CH3(CH2)nCH2OH Linear n = 4 to 20 CH3(CH2)nCHCH2OH 2-Alkyl branched n + m = 3 to 18, and m is predominantly (CH2)mCH3 = 0. Present in essentially-linear alcohols CH3(CH2)nCH(CH2)mOH Other-methyl branching n + m= 9 or 10 CH3 Present in essentially-linear Fischer- Tropsch derived alcohols CH3(CH2)7CH=CH(CH2)7CH2O Unsaturated H 9-Z unsaturated components are present in some commercial products. -

United States Patent Office Patented Nov
3,221,026 United States Patent Office Patented Nov. 30, 1965 2 3,221,026 prepared by reaction of a dicyanoketene acetal of the SALTS OF 1,1-DCYANO-2,2,2-TRIALKOXY formula ETHANES Owen W. Webster, Wilmington, Del, assignor to E. I. du Pont de Nemours and Company, Wilmington, Del., a corporation of Delaware No Drawing. Filed Feb. 13, 1962, Ser. No. 172,875 wherein R2 and R3 have the meanings defined above in the 2 Claims. (C. 260-340.9) general formula for the products of this invention, with This invention relates to salts of polycyano compounds, one molar equivalent of an alkali metal alkoxide of an and more particularly, to salts of polycyanopolyalkoxy alcohol having 1-8 carbon atoms at a temperature below ethanes and a process for their preparation. 10° C., and preferably at a temperature between 0 and The salts are derivatives of tetracyanoethylene which -80° C., in the presence of an inert reaction medium, is a very reactive compound that has received considerable e.g., an excess of the alcohol from which the alkoxide is study during the last few years. A large number of new 5 derived, or an ether such as diethyl ether, dioxane, tetra and valuable compounds have been prepared from it, and hydrofuran, ethylene glycol dimethyl ether and the like. now a new class of polycyano compounds is provided by As in the case of the reaction starting with tetracyano the present invention. ethylene, the reaction mixture in this case should also The novel compounds of this invention are salts of the be anhydrous to obtain the best results. -

Synthesis of Novel Single-Source Precursors for CVD of Mixed-Metal Tungsten Oxide
Synthesis of novel single-source precursors for CVD of mixed-metal tungsten oxide Hamid Choujaa A thesis submitted for the degree of Doctor of Philosophy University of Bath Department of Chemistry March 2008 COPYRIGHT Attention is drawn to the fact that copyright of this thesis rests with its author. This copy of the thesis has been supplied on condition that anyone who consults it is understood to recognize that its copyright rests with its author and that no quotation the thesis and no information derived from it may be published without the prior written consent of the author. This thesis may be made available for consultation within the University Library and may be photocopied or lent to other libraries for the purposes of consultation. TABLE OF CONTENTS Abstract ....................................................................................................................................... i Acknowledgements .................................................................................................................... iii Abbreviations and Acronyms .................................................................................................... iv 1. INTRODUCTION .................................................................................................................. 1 1.1 Generality about tungsten(VI) oxide ............................................................................. 1 1.1.1 The different lattice structures of tungsten oxide ........................................... 1 1.1.2 Electronic and -

8.6 Acidity of Alcohols and Thiols 355
08_BRCLoudon_pgs5-1.qxd 12/8/08 11:05 AM Page 355 8.6 ACIDITY OF ALCOHOLS AND THIOLS 355 ural barrier to the passage of ions. However, the hydrocarbon surface of nonactin allows it to enter readily into, and pass through, membranes. Because nonactin binds and thus transports ions, the ion balance crucial to proper cell function is upset, and the cell dies. Ion Channels Ion channels, or “ion gates,” provide passageways for ions into and out of cells. (Recall that ions are not soluble in membrane phospholipids.) The flow of ions is essen- tial for the transmission of nerve impulses and for other biological processes. A typical chan- nel is a large protein molecule imbedded in a cell membrane. Through various mechanisms, ion channels can be opened or closed to regulate the concentration of ions in the interior of the cell. Ions do not diffuse passively through an open channel; rather, an open channel contains regions that bind a specific ion. Such an ion is bound specifically within the channel at one side of the membrane and is somehow expelled from the channel on the other side. Remark- ably, the structures of the ion-binding regions of these channels have much in common with the structures of ionophores such as nonactin. The first X-ray crystal structure of a potassium- ion channel was determined in 1998 by a team of scientists at Rockefeller University led by Prof. Roderick MacKinnon (b. 1956), who shared the 2003 Nobel Prize in Chemistry for this work. The interior of the channel contains binding sites for two potassium ions; these sites are oxygen-rich, much like the interior of nonactin.