United States Patent Office Patented Mar
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Cyclobutane Derivatives in Drug Discovery
Cyclobutane Derivatives in Drug Discovery Overview Key Points Unlike larger and conformationally flexible cycloalkanes, Cyclobutane adopts a rigid cyclobutane and cyclopropane have rigid conformations. Due to the ring strain, cyclobutane adopts a rigid puckered puckered conformation Offer ing advantages on (~30°) conformation. This unique architecture bestowed potency, selectivity and certain cyclobutane-containing drugs with unique pharmacokinetic (PK) properties. When applied appropriately, cyclobutyl profile. scaffolds may offer advantages on potency, selectivity and pharmacokinetic (PK) profile. Bridging Molecules for Innovative Medicines 1 PharmaBlock designs and Cyclobutane-containing Drugs synthesizes over 1846 At least four cyclobutane-containing drugs are currently on the market. cyclobutanes, and 497 Chemotherapy carboplatin (Paraplatin, 1) for treating ovarian cancer was cyclobutane products are prepared to lower the strong nephrotoxicity associated with cisplatin. By in stock. CLICK HERE to replacing cisplatin’s two chlorine atoms with cyclobutane-1,1-dicarboxylic find detailed product acid, carboplatin (1) has a much lower nephrotoxicity than cisplatin. On information on webpage. the other hand, Schering-Plough/Merck’s hepatitis C virus (HCV) NS3/4A protease inhibitor boceprevir (Victrelis, 2) also contains a cyclobutane group in its P1 region. It is 3- and 19-fold more potent than the 1 corresponding cyclopropyl and cyclopentyl analogues, respectively. Androgen receptor (AR) antagonist apalutamide (Erleada, 4) for treating castration-resistant prostate cancer (CRPC) has a spirocyclic cyclobutane scaffold. It is in the same series as enzalutamide (Xtandi, 3) discovered by Jung’s group at UCLA in the 2000s. The cyclobutyl- (4) and cyclopentyl- derivative have activities comparable to the dimethyl analogue although the corresponding six-, seven-, and eight-membered rings are slightly less 2 active. -

Recent Advances in the Total Synthesis of Cyclobutane-Containing Natural Products Cite This: Org
Volume 7 | Number 1 | 7 January 2020 ORGANIC CHEMISTRY FRONTIERS rsc.li/frontiers-organic ORGANIC CHEMISTRY FRONTIERS View Article Online REVIEW View Journal | View Issue Recent advances in the total synthesis of cyclobutane-containing natural products Cite this: Org. Chem. Front., 2020, 7, 136 Jinshan Li,†a Kai Gao, †a Ming Bianb and Hanfeng Ding *a,c Complex natural products bearing strained cyclobutane subunits, including terpenoids, alkaloids and steroids, not only display fascinating architectures, but also show potent biological activities. Due to their unique structures as critical core skeletons in these molecules, a variety of new strategies for the con- Received 24th September 2019, struction of cyclobutane rings have greatly emerged during the last decade. In this review, we wish to Accepted 11th November 2019 summarize the recent progress in the cyclobutane-containing natural product synthesis with an emphasis DOI: 10.1039/c9qo01178a on disconnection tactics employed to forge the four-membered rings, aiming to provide a complement rsc.li/frontiers-organic to existing reviews. 1. Introduction stereoselectively, poses significant challenges in synthetic chemistry. On the other hand, cyclobutanes readily undergo a In the class of strained carbocycles, cyclobutanes have been number of ring-opening reactions by virtue of their tendency known as intriguing structural motifs for more than one to release inherent strain energies. In some cases, however, century but remained relatively less explored in parallel with striking ring strains can be dramatically reduced by the instal- their homologues.1 Due to the highly strained ring systems (ca. lation of a gem-dialkyl substituent (through the Thorpe–Ingold − 26.7 kcal mol 1), construction of cyclobutane rings, especially effect),2 a carbonyl group, a heteroatom, or other functional- ities (Fig. -

United States Patent Office Patented Nov
3,221,026 United States Patent Office Patented Nov. 30, 1965 2 3,221,026 prepared by reaction of a dicyanoketene acetal of the SALTS OF 1,1-DCYANO-2,2,2-TRIALKOXY formula ETHANES Owen W. Webster, Wilmington, Del, assignor to E. I. du Pont de Nemours and Company, Wilmington, Del., a corporation of Delaware No Drawing. Filed Feb. 13, 1962, Ser. No. 172,875 wherein R2 and R3 have the meanings defined above in the 2 Claims. (C. 260-340.9) general formula for the products of this invention, with This invention relates to salts of polycyano compounds, one molar equivalent of an alkali metal alkoxide of an and more particularly, to salts of polycyanopolyalkoxy alcohol having 1-8 carbon atoms at a temperature below ethanes and a process for their preparation. 10° C., and preferably at a temperature between 0 and The salts are derivatives of tetracyanoethylene which -80° C., in the presence of an inert reaction medium, is a very reactive compound that has received considerable e.g., an excess of the alcohol from which the alkoxide is study during the last few years. A large number of new 5 derived, or an ether such as diethyl ether, dioxane, tetra and valuable compounds have been prepared from it, and hydrofuran, ethylene glycol dimethyl ether and the like. now a new class of polycyano compounds is provided by As in the case of the reaction starting with tetracyano the present invention. ethylene, the reaction mixture in this case should also The novel compounds of this invention are salts of the be anhydrous to obtain the best results. -

Synthesis of Novel Single-Source Precursors for CVD of Mixed-Metal Tungsten Oxide
Synthesis of novel single-source precursors for CVD of mixed-metal tungsten oxide Hamid Choujaa A thesis submitted for the degree of Doctor of Philosophy University of Bath Department of Chemistry March 2008 COPYRIGHT Attention is drawn to the fact that copyright of this thesis rests with its author. This copy of the thesis has been supplied on condition that anyone who consults it is understood to recognize that its copyright rests with its author and that no quotation the thesis and no information derived from it may be published without the prior written consent of the author. This thesis may be made available for consultation within the University Library and may be photocopied or lent to other libraries for the purposes of consultation. TABLE OF CONTENTS Abstract ....................................................................................................................................... i Acknowledgements .................................................................................................................... iii Abbreviations and Acronyms .................................................................................................... iv 1. INTRODUCTION .................................................................................................................. 1 1.1 Generality about tungsten(VI) oxide ............................................................................. 1 1.1.1 The different lattice structures of tungsten oxide ........................................... 1 1.1.2 Electronic and -

8.6 Acidity of Alcohols and Thiols 355
08_BRCLoudon_pgs5-1.qxd 12/8/08 11:05 AM Page 355 8.6 ACIDITY OF ALCOHOLS AND THIOLS 355 ural barrier to the passage of ions. However, the hydrocarbon surface of nonactin allows it to enter readily into, and pass through, membranes. Because nonactin binds and thus transports ions, the ion balance crucial to proper cell function is upset, and the cell dies. Ion Channels Ion channels, or “ion gates,” provide passageways for ions into and out of cells. (Recall that ions are not soluble in membrane phospholipids.) The flow of ions is essen- tial for the transmission of nerve impulses and for other biological processes. A typical chan- nel is a large protein molecule imbedded in a cell membrane. Through various mechanisms, ion channels can be opened or closed to regulate the concentration of ions in the interior of the cell. Ions do not diffuse passively through an open channel; rather, an open channel contains regions that bind a specific ion. Such an ion is bound specifically within the channel at one side of the membrane and is somehow expelled from the channel on the other side. Remark- ably, the structures of the ion-binding regions of these channels have much in common with the structures of ionophores such as nonactin. The first X-ray crystal structure of a potassium- ion channel was determined in 1998 by a team of scientists at Rockefeller University led by Prof. Roderick MacKinnon (b. 1956), who shared the 2003 Nobel Prize in Chemistry for this work. The interior of the channel contains binding sites for two potassium ions; these sites are oxygen-rich, much like the interior of nonactin. -

Nitric Oxide Activation Facilitated by Cooperative Multimetallic Electron Transfer Within an Iron- Cite This: Chem
Chemical Science View Article Online EDGE ARTICLE View Journal | View Issue Nitric oxide activation facilitated by cooperative multimetallic electron transfer within an iron- Cite this: Chem. Sci.,2018,9,6379 – † All publication charges for this article functionalized polyoxovanadate alkoxide cluster have been paid for by the Royal Society a a a a b of Chemistry F. Li, R. L. Meyer, S. H. Carpenter, L. E. VanGelder, A. W. Nichols, C. W. Machan, b M. L. Neidiga and E. M. Matson *a A series of NO-bound, iron-functionalized polyoxovanadate–alkoxide (FePOV–alkoxide) clusters have been synthesized, providing insight into the role of multimetallic constructs in the coordination and activation of a substrate. Upon exposure of the heterometallic cluster to NO, the vanadium-oxide metalloligand is oxidized by a single electron, shuttling the reducing equivalent to the {FeNO} subunit to 7 V IV form a {FeNO} species. Four NO-bound clusters with electronic distributions ranging from [V3V2 ] Received 1st March 2018 {FeNO}7 to [VIV]{FeNO}7 have been synthesized, and characterized via 1H NMR, infrared, and electronic Accepted 30th June 2018 5 absorption spectroscopies. The ability of the FePOV–alkoxide cluster to store reducing equivalents in the DOI: 10.1039/c8sc00987b Creative Commons Attribution 3.0 Unported Licence. metalloligand for substrate coordination and activation highlights the ultility of the metal-oxide scaffold rsc.li/chemical-science as a redox reservoir. Introduction The activation of NO requires the simultaneous transfer of multiple electrons and protons to the substrate. In nature, The chemical reactivity of nitric oxide (NO) has captivated the eld similar chemical transformations of gaseous substrates (e.g. -

Direct Synthesis of Some Significant Metal Alkoxides
SD0000032 DIRECT SYNTHESIS OF SOME SIGNIFICANT METAL ALKOXI'DE BVYU EMI1JG A THESIS SUBMITTED FOR THE DEGREE OF Mi.Sc. IN CHEMISTRY SUPERVISOR: Dr. O.Y.OMER DEPARTMENT OF CHEMISTRY FACULTY OF EDUCATION UNIVERSITY OF KHARTOUM NOVEMBER, 1998 31/28 DISCLAIMER Portions of this document may be illegible in electronic image products. Images are produced from the best available original document. Please be aware that all of the Missing Pages in this document were originally blank pages Dedication To my three children: Regina, Maria and Samuel CONTENTS Page Dedication i Contents ii List of Tables v List of Figures vii Acknowledgement viii Abstract (Arabic) ix Abstract (English) x CHAPTER 1.0 CHAPTER ONE - INTRODUCTION 1 2.0 CHAPTER TWO - LITERATURE REVIEW 5 2.1 Introduction to Literature Review 5 2.2 Definition of metal alkoxides 5 2.3 Metal elements and (heir chemistry 8 2.3.1 Sodium metal 8 2.3.2 Magnesium metal 12 2.3.3 Aluminium metal 16 2.3.3.1 Hydrolysis of aluminium compounds 20 2.3.4 Tin metal 21 2.4 Preparative methods and uses ofalkoxides ofNa, Mg, Al & Sn. 25 2.4.1 Sodium alkoxide 25 2.4.2 Mamiesium alkoxide 26 2.4.3 Aluminium alkoxide 27 2.4.4. Tin alkoxide 30 2.5 General properties of metal alkoxides 31 2.5.1 1 lydrolysis in metal alkoxide 34 3.0 CHAPTER THREE - MATERIALS AND EXPERIMENTAL PROCEDURE 36 3.1 General procedures 36 3.1.1 Start ing material s 3 6 3.1. I.I Apparatus 36 3.1.1.2 Dry ethanol and isopropanol 36 3.1.1.3 Na, Mg, Al & Sn metals 36 3.1.2 Infrared spectra (Ir) 37 3.2 Reactions procedures 37 3.2.1 Reaction between sodium metal and absolute ethanol 37 3.2.2 Reaction of magnesium metal with absolute ethanol 37 3.2.3 Reaction of magnesium mclal with absolute ethanol using mercury (11) chloride catalyst. -

United States Patent Office Patented May 7, 1963
3,088,959 United States Patent Office Patented May 7, 1963 1. 2 or grouping of carbon atoms which is present in cyclo 3,088,959 pentadiene. This grouping is illustrated as PROCESS OF MAKENG CYCLOPENTADEENY NECKEL, NTROSYL COMPOUNDS Robert D. Feltham, Joseph F. Anzenberger, azad Jonatian T. Carrie, Pittsburgh, Pa., assignors to The Interaa tional Nickel Company, Inc., New York, N.Y., a corpo ration of Delaware No Drawing. FiRed Sept. 1, 1960, Ser. No. 53,374 The substituent groups on the cyclopentadiene moiety 6 Clains. (C. 260-439) 0. indicated as R, R2, R3, R and R5 are any one or more The present invention relates to the production of of hydrogen atoms, halogen atoms and/or organic groups nickel compounds and, more particularly, to the produc such as aliphatic groups, aromatic groups, alicyclic groups, tion of nickel nitrosyl compounds containing a group etc. The substituent groups can also bond at two posi having the cyclopentadienyl moiety. tions. Where this occurs, groups can substitute for adja Compounds such as cyclopentadienylnickel nitrosyl, 5 cent R groups, e.g., Ra and R3 and/or R4 and R5 to form methylcyclopentadienylnickel nitrosyl and other complex indene and other condensed ring structures. nitrosyl compounds containing a cyclopentadienyl-type As mentioned hereinbefore, when carrying out the proc group have been made. Such compounds have use as ess of the present invention, the reactants are reacted in gasoline additives. When such use is contemplated, it is the presence of a base. The base can advantageously be economically imperative that the compounds be produced 20 a nitrogen base or a phosphorus base or an alkoxide of a in good yield from the most readily available and inex metal having a strong hydroxide. -
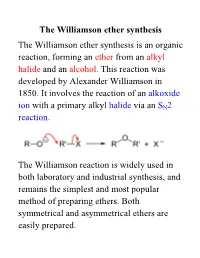
Williamson Ether Synthesis the Williamson Ether Synthesis Is an Organic Reaction, Forming an Ether from an Alkyl Halide and an Alcohol
The Williamson ether synthesis The Williamson ether synthesis is an organic reaction, forming an ether from an alkyl halide and an alcohol. This reaction was developed by Alexander Williamson in 1850. It involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction. The Williamson reaction is widely used in both laboratory and industrial synthesis, and remains the simplest and most popular method of preparing ethers. Both symmetrical and asymmetrical ethers are easily prepared. The reaction for this week: an example of a Williamson ether synthesis acetaminophen ethyl iodide phenacetin starting material reagent product Phenacetin may be synthesized as an example of the Williamson ether synthesis The first synthesis of phenacetin was reported in 1878 by Harmon Morse. Procedure 1. Weigh an Extra-Strength Tylenol tablet. Pulverize the tablet with mortar and pestle. Weigh out 0.22 g and place it in a dry 15-ml round-bottom flask along with 0.28 g of finely pulverized K2CO3 (mortar and pestle) and 3.0 mL of butanone. Carefully add 0.28 mL of ethyl iodide with a syringe. 2. Add a stir bar; attach a microscale water-cooled condenser to the flask. Heat the mixture under reflux directly on a hot plate at medium setting for 1 hour. In the meantime, obtain the IR of acetaminophen. 3. Turn off the heat. Allow the mixture to cool down. Add 4 mL of water to the flask and transfer its contents to a 16 x 125 mm test tube with a screw cap. Rinse round-bottom flask 4 times with 1 mL of tert-butyl methyl ether (BME) and add the rinsings to the test tube. -

Jailbreaking Benzene Dimers † Andrey Yu
Communication pubs.acs.org/JACS Jailbreaking Benzene Dimers † Andrey Yu. Rogachev, Xiao-Dong Wen, and Roald Hoffmann* Baker Laboratory, Department of Chemistry and Chemical Biology, Cornell University, Ithaca, New York 14853-1301, United States *S Supporting Information 5 and 6 were calculated as not much less stable than the known ABSTRACT: We suggest four new benzene dimers, dimers. (C6H6)2, all featuring one or more cyclohexadiene rings trans-fused to 4- or 6-membered rings. These hypothetical dimers are 50−99 kcal/mol less stable than two benzenes, but have computed activation energies to fragmentation ≥27 kcal/mol. A thorough search of potential escape routes was undertaken, through cyclobutane ring cleavage to 12-annulenes, sigmatropic 1,5-H-shifts, electrocyclic ring-openings of the 6-membered rings, and Diels−Alder dimerizations. Some channels for reaction emerge, but there is a reasonable chance that some of these new benzene dimers can be made. Apparently trans-bridging a butadiene 1,2 on a cyclobutane ring, with the latter’s freedom to pucker, is not as difficult as one n the course of thinking about benzene under gigapascal might think.11 Nor is it problematic for a butadiene to bridge the I pressure,1 we decided we might learn something from the gauche positions of an approximately staggered ethane. dimers of benzene, as signposts to the pressure-induced poly- No longer succumbing to the prejudice of avoiding trans- merization of the compound. To induce the benzenes to dimerize, bridging of a cyclobutane ring by a butadiene, from this point we brought two benzene molecules to an uncomfortably close we proceeded down a systematic (human, not machine) path, contact,2 and then let loose the geometry optimization of a 3 generating geometries for all reasonable cycloadducts. -

Phosphine-Catalyzed Additions of Nucleophiles and Electrophiles to Α
PHOSPHINE-CATALYZED ADDITIONS OF NUCLEOPHILES AND ELECTROPHILES TO α,β–UNSATURATED CARBONYL COMPOUNDS Reported by Michael Scott Bultman November 4, 2004 INTRODUCTION Organophosphorous compounds are becoming increasingly important in organic synthesis. Phosphines serve as precursors to phosphonium ylides in the Wittig reaction,1 and as nucleophilic triggers in the Mitsunobu2 and Staudinger3 reactions. In these processes, the phosphine is stoichiometrically consumed and converted into a phosphine oxide. Phosphines are also commonly used as ligands for transition metal-catalyzed reactions, to modulate reactivity and stereocontrol.4 On the other hand, the use of phosphines as nucleophilic catalysts for organic reactions has only gained attention in the last ten years. First reported by Rauhut and Currier in 1963,5 phosphine catalysis has since been reinvestigated after the phosphine ligands in some transition-metal-catalyzed reactions were found to be better catalysts than the metal/phosphine complexes alone!6 Phosphines are well suited for catalyzing the addition of both nucleophiles and electrophiles to electron deficient alkenes, alkynes, and allenes. Activation of these α,β-unsaturated carbonyl systems with the phosphine enables the formation of new bonds at the α-, β-, and γ-positions. This report will highlight these different modes of addition to α,β-unsaturated carbonyl systems under phosphine catalysis that allow for the formation of a wide array of products from a single class of substrates. GENERAL REACTIVITY OF PHOSPHINES Key characteristics required for successful nucleophilic catalysis lie in the balance of leaving group ability, nucleophilicity, and ease of ylid formation. Increasing leaving group ability can often be + correlated with decreasing basicity. -

Hydrocarbon Compounds 22
05_Chem_GRSW_Ch22.SE/TE 6/11/04 3:52 PM Page 237 Name ___________________________ Date ___________________ Class __________________ HYDROCARBON COMPOUNDS 22 SECTION 22.1 HYDROCARBONS (pages 693–701) This section describes the bonding in hydrocarbons and distinguishes straight-chain from branched-chain alkanes. It also provides rules for naming branch-chained alkanes. Organic Chemistry and Hydrocarbons (pages 693–694) 1. What is organic chemistry? It__________________________________________________ is the study of the chemistry of carbon compounds. 2. Organic compounds that contain only carbon and hydrogen are called ______________________hydrocarbons . 3. Is the following sentence true or false? Hydrogen atoms are the only atoms that can bond to the carbon atoms in a hydrocarbon. ______________________false 4. Circle the letter of each statement that is true about carbon’s ability to form bonds. a. Carbon atoms have four valence electrons. b. Carbon atoms always form three covalent bonds. c. Carbon atoms can form stable bonds with other carbon atoms. Alkanes (pages 694–699) 5. Is the following sentence true or false? Alkanes contain only single covalent bonds. ______________________true 6. What is the simplest alkane? ______________________methane 7. What are straight-chain alkanes?They ______________________________________________ contain any number of carbon atoms, one after another, in a chain. 8. The names of all alkanes end with the suffix ______________________-ane . Match the name of the straight-chain alkane with the number of carbon atoms it contains. _______d 9. nonane a. 3 _______a 10. propane b. 4 c _______ 11. heptane c. 7 © Pearson Education, Inc., publishing as Prentice Hall. All rights reserved. _______b 12. butane d. 9 13. The straight-chain alkanes form a(n) ______________________________homologous series because there is an incremental change of a CH2 group from one compound in the series to the next.