1 Chapter 3: Organic Compounds: Alkanes and Cycloalkanes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![C9-14 Aliphatic [2-25% Aromatic] Hydrocarbon Solvents Category SIAP](https://docslib.b-cdn.net/cover/2852/c9-14-aliphatic-2-25-aromatic-hydrocarbon-solvents-category-siap-12852.webp)
C9-14 Aliphatic [2-25% Aromatic] Hydrocarbon Solvents Category SIAP
CoCAM 2, 17-19 April 2012 BIAC/ICCA SIDS INITIAL ASSESSMENT PROFILE Chemical C -C Aliphatic [2-25% aromatic] Hydrocarbon Solvents Category Category 9 14 Substance Name CAS Number Stoddard solvent 8052-41-3 Chemical Names Kerosine, petroleum, hydrodesulfurized 64742-81-0 and CAS Naphtha, petroleum, hydrodesulfurized heavy 64742-82-1 Registry Solvent naphtha, petroleum, medium aliphatic 64742-88-7 Numbers Note: Substances in this category are also commonly known as mineral spirits, white spirits, or Stoddard solvent. CAS Number Chemical Description † 8052-41-3 Includes C8 to C14 branched, linear, and cyclic paraffins and aromatics (6 to 18%), <50ppmV benzene † 64742-81-0 Includes C9 to C14 branched, linear, and cyclic paraffins and aromatics (10 to Structural 25%), <100 ppmV benzene Formula † and CAS 64742-82-1 Includes C8 to C13 branched, linear, and cyclic paraffins and aromatics (15 to 25%), <100 ppmV benzene Registry † Numbers 64742-88-7 Includes C8 to C13 branched, linear, and cyclic paraffins and aromatics (14 to 20%), <50 ppmV benzene Individual category member substances are comprised of aliphatic hydrocarbon molecules whose carbon numbers range between C9 and C14; approximately 80% of the aliphatic constituents for a given substance fall within the C9-C14 carbon range and <100 ppmV benzene. In some instances, the carbon range of a test substance is more precisely defined in the test protocol. In these instances, the specific carbon range (e.g. C8-C10, C9-C10, etc.) will be specified in the SIAP. * It should be noted that other substances defined by the same CAS RNs may have boiling ranges outside the range of 143-254° C and that these substances are not covered by the category. -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -
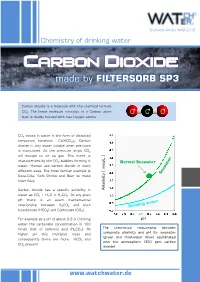
Carbon Dioxide - Made by FILTERSORB SP3
Technical Article MAR 2015 Chemistry of drinking water Carbon dioxide - made by FILTERSORB SP3 Carbon dioxide is a molecule with the chemical formula CO2. The linear molecule consists of a Carbon atom O C O that is doubly bonded with two Oxygen atoms. CO2 exists in water in the form of dissolved temporary hardness Ca(HCO3)2. Carbon dioxide is only water soluble when pressure is maintained. As the pressure drops CO2 will escape to air as gas. This event is characterized by the CO2 bubbles forming in /L ] Normal Seawater water. Human use carbon dioxide in many meq different ways. The most familiar example is Coca-Cola, Soft Drinks and Beer to make them fizzy. Carbon dioxide has a specific solubility in [ Alkalinity water as CO2 + H2O ⇌ H2CO3. At any given pH there is an exact mathematical relationship between H2CO3 and both bicarbonate (HCO3) and Carbonate (CO3). For example at a pH of about 9.3 in Drinking pH water the carbonate concentration is 100 The theoretical relationship between times that of carbonic acid (H2CO3). At carbonate alkalinity and pH for seawater higher pH this multiplier rises and (green and freshwater (blue) equilibrated consequently there are more HCO and 3 with the atmosphere (350 ppm carbon CO present 3 dioxide) www.watchwater.de Water ® Technology FILTERSORB SP3 WATCH WATER & Chemicals Making the healthiest water How SP3 Water functions in human body ? Answer: Carbon dioxide is a waste product of the around these hollow spaces will spasm and respiratory system, and of several other constrict. chemical reactions in the body such as the Transportation of oxygen to the tissues: Oxygen creation of ATP. -

The Relative Rates of Thiol–Thioester Exchange and Hydrolysis for Alkyl and Aryl Thioalkanoates in Water
Orig Life Evol Biosph (2011) 41:399–412 DOI 10.1007/s11084-011-9243-4 PREBIOTIC CHEMISTRY The Relative Rates of Thiol–Thioester Exchange and Hydrolysis for Alkyl and Aryl Thioalkanoates in Water Paul J. Bracher & Phillip W. Snyder & Brooks R. Bohall & George M. Whitesides Received: 14 April 2011 /Accepted: 16 June 2011 / Published online: 5 July 2011 # Springer Science+Business Media B.V. 2011 Abstract This article reports rate constants for thiol–thioester exchange (kex), and for acid- mediated (ka), base-mediated (kb), and pH-independent (kw) hydrolysis of S-methyl thioacetate and S-phenyl 5-dimethylamino-5-oxo-thiopentanoate—model alkyl and aryl thioalkanoates, respectively—in water. Reactions such as thiol–thioester exchange or aminolysis could have generated molecular complexity on early Earth, but for thioesters to have played important roles in the origin of life, constructive reactions would have needed to compete effectively with hydrolysis under prebiotic conditions. Knowledge of the kinetics of competition between exchange and hydrolysis is also useful in the optimization of systems where exchange is used in applications such as self-assembly or reversible binding. For the alkyl thioester S-methyl thioacetate, which has been synthesized in −5 −1 −1 −1 −1 −1 simulated prebiotic hydrothermal vents, ka = 1.5×10 M s , kb = 1.6×10 M s , and −8 −1 kw = 3.6×10 s . At pH 7 and 23°C, the half-life for hydrolysis is 155 days. The second- order rate constant for thiol–thioester exchange between S-methyl thioacetate and 2- −1 −1 sulfonatoethanethiolate is kex = 1.7 M s . -

Chapter 7, Haloalkanes, Properties and Substitution Reactions of Haloalkanes Table of Contents 1
Chapter 7, Haloalkanes, Properties and Substitution Reactions of Haloalkanes Table of Contents 1. Alkyl Halides (Haloalkane) 2. Nucleophilic Substitution Reactions (SNX, X=1 or 2) 3. Nucleophiles (Acid-Base Chemistry, pka) 4. Leaving Groups (Acid-Base Chemistry, pka) 5. Kinetics of a Nucleophilic Substitution Reaction: An SN2 Reaction 6. A Mechanism for the SN2 Reaction 7. The Stereochemistry of SN2 Reactions 8. A Mechanism for the SN1 Reaction 9. Carbocations, SN1, E1 10. Stereochemistry of SN1 Reactions 11. Factors Affecting the Rates of SN1 and SN2 Reactions 12. --Eliminations, E1 and E2 13. E2 and E1 mechanisms and product predictions In this chapter we will consider: What groups can be replaced (i.e., substituted) or eliminated The various mechanisms by which such processes occur The conditions that can promote such reactions Alkyl Halides (Haloalkane) An alkyl halide has a halogen atom bonded to an sp3-hybridized (tetrahedral) carbon atom The carbon–chlorine and carbon– bromine bonds are polarized because the halogen is more electronegative than carbon The carbon-iodine bond do not have a per- manent dipole, the bond is easily polarizable Iodine is a good leaving group due to its polarizability, i.e. its ability to stabilize a charge due to its large atomic size Generally, a carbon-halogen bond is polar with a partial positive () charge on the carbon and partial negative () charge on the halogen C X X = Cl, Br, I Different Types of Organic Halides Alkyl halides (haloalkanes) sp3-hybridized Attached to Attached to Attached -

A Complete Guide to Benzene
A Complete Guide to Benzene Benzene is an important organic chemical compound with the chemical formula C6H6. The benzene molecule is composed of six carbon atoms joined in a ring with one hydrogen atom attached to each. As it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of crude oil and is one of the elementary petrochemicals. Due to the cyclic continuous pi bond between the carbon atoms, benzene is classed as an aromatic hydrocarbon, the second [n]- annulene ([6]-annulene). It is sometimes abbreviated Ph–H. Benzene is a colourless and highly flammable liquid with a sweet smell. Source: Wikipedia Protecting people and the environment Protecting both people and the environment whilst meeting the operational needs of your business is very important and, if you have operations in the UK you will be well aware of the requirements of the CoSHH Regulations1 and likewise the Code of Federal Regulations (CFR) in the US2. Similar legislation exists worldwide, the common theme being an onus on hazard identification, risk assessment and the provision of appropriate control measures (bearing in mind the hierarchy of controls) as well as health surveillance in most cases. And whilst toxic gasses such as hydrogen sulphide and carbon monoxide are a major concern because they pose an immediate (acute) danger to life, long term exposure to relatively low level concentrations of other gasses or vapours such as volatile organic compounds (VOC) are of equal importance because of the chronic illnesses that can result from that ongoing exposure. Benzene, a common VOC Organic means the chemistry of carbon based compounds, which are substances that results from a combination of two or more different chemical elements. -

Cyclobutane Derivatives in Drug Discovery
Cyclobutane Derivatives in Drug Discovery Overview Key Points Unlike larger and conformationally flexible cycloalkanes, Cyclobutane adopts a rigid cyclobutane and cyclopropane have rigid conformations. Due to the ring strain, cyclobutane adopts a rigid puckered puckered conformation Offer ing advantages on (~30°) conformation. This unique architecture bestowed potency, selectivity and certain cyclobutane-containing drugs with unique pharmacokinetic (PK) properties. When applied appropriately, cyclobutyl profile. scaffolds may offer advantages on potency, selectivity and pharmacokinetic (PK) profile. Bridging Molecules for Innovative Medicines 1 PharmaBlock designs and Cyclobutane-containing Drugs synthesizes over 1846 At least four cyclobutane-containing drugs are currently on the market. cyclobutanes, and 497 Chemotherapy carboplatin (Paraplatin, 1) for treating ovarian cancer was cyclobutane products are prepared to lower the strong nephrotoxicity associated with cisplatin. By in stock. CLICK HERE to replacing cisplatin’s two chlorine atoms with cyclobutane-1,1-dicarboxylic find detailed product acid, carboplatin (1) has a much lower nephrotoxicity than cisplatin. On information on webpage. the other hand, Schering-Plough/Merck’s hepatitis C virus (HCV) NS3/4A protease inhibitor boceprevir (Victrelis, 2) also contains a cyclobutane group in its P1 region. It is 3- and 19-fold more potent than the 1 corresponding cyclopropyl and cyclopentyl analogues, respectively. Androgen receptor (AR) antagonist apalutamide (Erleada, 4) for treating castration-resistant prostate cancer (CRPC) has a spirocyclic cyclobutane scaffold. It is in the same series as enzalutamide (Xtandi, 3) discovered by Jung’s group at UCLA in the 2000s. The cyclobutyl- (4) and cyclopentyl- derivative have activities comparable to the dimethyl analogue although the corresponding six-, seven-, and eight-membered rings are slightly less 2 active. -

Implications for Extraterrestrial Hydrocarbon Chemistry: Analysis Of
The Astrophysical Journal, 889:3 (26pp), 2020 January 20 https://doi.org/10.3847/1538-4357/ab616c © 2020. The American Astronomical Society. All rights reserved. Implications for Extraterrestrial Hydrocarbon Chemistry: Analysis of Acetylene (C2H2) and D2-acetylene (C2D2) Ices Exposed to Ionizing Radiation via Ultraviolet–Visible Spectroscopy, Infrared Spectroscopy, and Reflectron Time-of-flight Mass Spectrometry Matthew J. Abplanalp1,2 and Ralf I. Kaiser1,2 1 W. M. Keck Research Laboratory in Astrochemistry, University of Hawaii at Manoa, Honolulu, HI 96822, USA 2 Department of Chemistry, University of Hawaii at Manoa, Honolulu, HI 96822, USA Received 2019 October 4; revised 2019 December 7; accepted 2019 December 10; published 2020 January 20 Abstract The processing of the simple hydrocarbon ice, acetylene (C2H2/C2D2), via energetic electrons, thus simulating the processes in the track of galactic cosmic-ray particles penetrating solid matter, was carried out in an ultrahigh vacuum surface apparatus. The chemical evolution of the ices was monitored online and in situ utilizing Fourier transform infrared spectroscopy (FTIR) and ultraviolet–visible spectroscopy and, during temperature programmed desorption, via a quadrupole mass spectrometer with an electron impact ionization source (EI-QMS) and a reflectron time-of-flight mass spectrometer utilizing single-photon photoionization (SPI-ReTOF-MS) along with resonance-enhanced multiphoton photoionization (REMPI-ReTOF-MS). The confirmation of previous in situ studies of ethylene ice irradiation -

First Principles Prediction of Thermodynamic Properties
2 First Principles Prediction of Thermodynamic Properties Hélio F. Dos Santos and Wagner B. De Almeida NEQC: Núcleo de Estudos em Química Computacional, Departamento de Química, ICE Universidade Federal de Juiz de Fora (UFJF), Campus Universitário Martelos, Juiz de Fora LQC-MM: Laboratório de Química Computacional e Modelagem Molecular Departamento de Química, ICEx, Universidade Federal de Minas Gerais (UFMG) Campus Universitário, Pampulha, Belo Horizonte Brazil 1. Introduction The determination of the molecular structure is undoubtedly an important issue in chemistry. The knowledge of the tridimensional structure allows the understanding and prediction of the chemical-physics properties and the potential applications of the resulting material. Nevertheless, even for a pure substance, the structure and measured properties reflect the behavior of many distinct geometries (conformers) averaged by the Boltzmann distribution. In general, for flexible molecules, several conformers can be found and the analysis of the physical and chemical properties of these isomers is known as conformational analysis (Eliel, 1965). In most of the cases, the conformational processes are associated with small rotational barriers around single bonds, and this fact often leads to mixtures, in which many conformations may exist in equilibrium (Franklin & Feltkamp, 1965). Therefore, the determination of temperature-dependent conformational population is very much welcomed in conformational analysis studies carried out by both experimentalists and theoreticians. -

Comparing Models for Measuring Ring Strain of Common Cycloalkanes
The Corinthian Volume 6 Article 4 2004 Comparing Models for Measuring Ring Strain of Common Cycloalkanes Brad A. Hobbs Georgia College Follow this and additional works at: https://kb.gcsu.edu/thecorinthian Part of the Chemistry Commons Recommended Citation Hobbs, Brad A. (2004) "Comparing Models for Measuring Ring Strain of Common Cycloalkanes," The Corinthian: Vol. 6 , Article 4. Available at: https://kb.gcsu.edu/thecorinthian/vol6/iss1/4 This Article is brought to you for free and open access by the Undergraduate Research at Knowledge Box. It has been accepted for inclusion in The Corinthian by an authorized editor of Knowledge Box. Campring Models for Measuring Ring Strain of Common Cycloalkanes Comparing Models for Measuring R..ing Strain of Common Cycloalkanes Brad A. Hobbs Dr. Kenneth C. McGill Chemistry Major Faculty Sponsor Introduction The number of carbon atoms bonded in the ring of a cycloalkane has a large effect on its energy. A molecule's energy has a vast impact on its stability. Determining the most stable form of a molecule is a usefol technique in the world of chemistry. One of the major factors that influ ence the energy (stability) of cycloalkanes is the molecule's ring strain. Ring strain is normally viewed as being directly proportional to the insta bility of a molecule. It is defined as a type of potential energy within the cyclic molecule, and is determined by the level of "strain" between the bonds of cycloalkanes. For example, propane has tl1e highest ring strain of all cycloalkanes. Each of propane's carbon atoms is sp3-hybridized. -

Recent Advances in the Total Synthesis of Cyclobutane-Containing Natural Products Cite This: Org
Volume 7 | Number 1 | 7 January 2020 ORGANIC CHEMISTRY FRONTIERS rsc.li/frontiers-organic ORGANIC CHEMISTRY FRONTIERS View Article Online REVIEW View Journal | View Issue Recent advances in the total synthesis of cyclobutane-containing natural products Cite this: Org. Chem. Front., 2020, 7, 136 Jinshan Li,†a Kai Gao, †a Ming Bianb and Hanfeng Ding *a,c Complex natural products bearing strained cyclobutane subunits, including terpenoids, alkaloids and steroids, not only display fascinating architectures, but also show potent biological activities. Due to their unique structures as critical core skeletons in these molecules, a variety of new strategies for the con- Received 24th September 2019, struction of cyclobutane rings have greatly emerged during the last decade. In this review, we wish to Accepted 11th November 2019 summarize the recent progress in the cyclobutane-containing natural product synthesis with an emphasis DOI: 10.1039/c9qo01178a on disconnection tactics employed to forge the four-membered rings, aiming to provide a complement rsc.li/frontiers-organic to existing reviews. 1. Introduction stereoselectively, poses significant challenges in synthetic chemistry. On the other hand, cyclobutanes readily undergo a In the class of strained carbocycles, cyclobutanes have been number of ring-opening reactions by virtue of their tendency known as intriguing structural motifs for more than one to release inherent strain energies. In some cases, however, century but remained relatively less explored in parallel with striking ring strains can be dramatically reduced by the instal- their homologues.1 Due to the highly strained ring systems (ca. lation of a gem-dialkyl substituent (through the Thorpe–Ingold − 26.7 kcal mol 1), construction of cyclobutane rings, especially effect),2 a carbonyl group, a heteroatom, or other functional- ities (Fig. -

Chemistry 0310 - Organic Chemistry 1 Chapter 3
Dr. Peter Wipf Chemistry 0310 - Organic Chemistry 1 Chapter 3. Reactions of Alkanes The heterolysis of covalent bonds yields anions and cations, whereas the homolysis creates radicals. Radicals are species with unpaired electrons that react mostly as electrophiles, seeking a single electron to complete their octet. Free radicals are important reaction intermediates and are formed in initiation reactions under conditions that cause the homolytic cleavage of bonds. In propagation steps, radicals abstract hydrogen or halogen atoms to create new radicals. Combinations of radicals are rare due to the low concentration of these reactive intermediates and result in termination of the radical chain. !CHAIN REACTION SUMMARY reactant product initiation PhCH3 HCl Cl 2 h DH = -16 kcal/mol chain-carrying intermediates n o r D (low concentrations) PhCH2 . Cl . propagation PhCH2 . or Cl . PhCH . 2 DH = -15 kcal/mol or Cl . PhCH2Cl or PhCH CH Ph PhCH2Cl Cl2 2 2 PhCH2Cl or Cl2 termination product reactant termination Alkanes are converted to alkyl halides by free radical halogenation reactions. The relative stability of radicals is increased by conjugation and hyperconjugation: R H H H . CH2 > R C . > R C . > H C . > H C . R R R H Oxygen is a diradical. In the presence of free-radical initiators such as metal salts, organic compounds and oxygen react to give hydroperoxides. These autoxidation reactions are responsible for the degradation reactions of oils, fatty acids, and other biological substances when exposed to air. Antioxidants such as hindered phenols are important food additives. Vitamins E and C are biological antioxidants. Radical chain reactions of chlorinated fluorocarbons in the stratosphere are responsible for the "ozone hole".