Chapter 7, Haloalkanes, Properties and Substitution Reactions of Haloalkanes Table of Contents 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Products from Reactions of Carbon Nucleophiles and Carbon
14C synthesis strategies, Chem 315/316 / Beauchamp 1 Products from reactions of carbon nucleophiles and carbon electrophiles used in the 14C Game and our course: Carbon and hydrogen nucleophiles Ph R Ph Al H N R Li R (MgBr) P Li AlH R Cu Li Na CN RCC Na Ph 4 Li Carbon 2 H C R organolithium organolithium cyanide acetylides 2 ylid Na BH4 diisobutylaluminium lithium diisopropy- electrophiles cuprates (LAH) o reagents reagents Wittig reagents hydride (DIBAH) amide (LDA), -78 C H C Br 3 not useful not useful 2 RX coupling nitrilesalkynes not useful alkyls alkyls not useful methyl RX reaction R Br not useful not useful 2 RX coupling nitriles alkynes not useful alkyls alkyls not useful primary RX reaction R 2 RX coupling nitriles alkyls not useful E2 not useful alkyls not useful not useful reaction R Br secondary RX O 1o ROH 1o ROH 1o ROH 1o ROH not useful 1o ROH o not useful not useful 1o ROH 1 ROH ethylene oxide nitriles alkynes O o o o o o o 2 ROH 2 ROH 2 ROH 2 ROH 2o ROH 2 ROH 2 ROH not useful not useful E2, make nitriles alkynes allylic alcohols propylene oxide O o 3 ROH 3o ROH o 3o ROH o not useful o 3o ROH not useful E2, make 3 ROH 3 ROH 3 ROH allylic alcohols nitriles alkynes isobutylene oxide O o o specific methanol methanol C 1 ROH 1 ROH not used cyanohydrin 1o ROH not useful not useful in our course alkenes H H alkynes methanal O specific o enolate o o cyanohydrin o 1 ROH o C 2 ROH 2 ROH not useful 2 ROH alkenes 1 ROH not useful chemistry R H alkynes simple aldehydes O cyanohydrin o unless specific o enolate C 3 ROH 3o ROH o 2o ROH -

The Relative Rates of Thiol–Thioester Exchange and Hydrolysis for Alkyl and Aryl Thioalkanoates in Water
Orig Life Evol Biosph (2011) 41:399–412 DOI 10.1007/s11084-011-9243-4 PREBIOTIC CHEMISTRY The Relative Rates of Thiol–Thioester Exchange and Hydrolysis for Alkyl and Aryl Thioalkanoates in Water Paul J. Bracher & Phillip W. Snyder & Brooks R. Bohall & George M. Whitesides Received: 14 April 2011 /Accepted: 16 June 2011 / Published online: 5 July 2011 # Springer Science+Business Media B.V. 2011 Abstract This article reports rate constants for thiol–thioester exchange (kex), and for acid- mediated (ka), base-mediated (kb), and pH-independent (kw) hydrolysis of S-methyl thioacetate and S-phenyl 5-dimethylamino-5-oxo-thiopentanoate—model alkyl and aryl thioalkanoates, respectively—in water. Reactions such as thiol–thioester exchange or aminolysis could have generated molecular complexity on early Earth, but for thioesters to have played important roles in the origin of life, constructive reactions would have needed to compete effectively with hydrolysis under prebiotic conditions. Knowledge of the kinetics of competition between exchange and hydrolysis is also useful in the optimization of systems where exchange is used in applications such as self-assembly or reversible binding. For the alkyl thioester S-methyl thioacetate, which has been synthesized in −5 −1 −1 −1 −1 −1 simulated prebiotic hydrothermal vents, ka = 1.5×10 M s , kb = 1.6×10 M s , and −8 −1 kw = 3.6×10 s . At pH 7 and 23°C, the half-life for hydrolysis is 155 days. The second- order rate constant for thiol–thioester exchange between S-methyl thioacetate and 2- −1 −1 sulfonatoethanethiolate is kex = 1.7 M s . -

Reactions of Benzene & Its Derivatives
Organic Lecture Series ReactionsReactions ofof BenzeneBenzene && ItsIts DerivativesDerivatives Chapter 22 1 Organic Lecture Series Reactions of Benzene The most characteristic reaction of aromatic compounds is substitution at a ring carbon: Halogenation: FeCl3 H + Cl2 Cl + HCl Chlorobenzene Nitration: H2 SO4 HNO+ HNO3 2 + H2 O Nitrobenzene 2 Organic Lecture Series Reactions of Benzene Sulfonation: H 2 SO4 HSO+ SO3 3 H Benzenesulfonic acid Alkylation: AlX3 H + RX R + HX An alkylbenzene Acylation: O O AlX H + RCX 3 CR + HX An acylbenzene 3 Organic Lecture Series Carbon-Carbon Bond Formations: R RCl AlCl3 Arenes Alkylbenzenes 4 Organic Lecture Series Electrophilic Aromatic Substitution • Electrophilic aromatic substitution: a reaction in which a hydrogen atom of an aromatic ring is replaced by an electrophile H E + + + E + H • In this section: – several common types of electrophiles – how each is generated – the mechanism by which each replaces hydrogen 5 Organic Lecture Series EAS: General Mechanism • A general mechanism slow, rate + determining H Step 1: H + E+ E El e ctro - Resonance-stabilized phile cation intermediate + H fast Step 2: E + H+ E • Key question: What is the electrophile and how is it generated? 6 Organic Lecture Series + + 7 Organic Lecture Series Chlorination Step 1: formation of a chloronium ion Cl Cl + + - - Cl Cl+ Fe Cl Cl Cl Fe Cl Cl Fe Cl4 Cl Cl Chlorine Ferric chloride A molecular complex An ion pair (a Lewis (a Lewis with a positive charge containing a base) acid) on ch lorine ch loronium ion Step 2: attack of -

Classifying Halogenoalkanes Reactions Of
10 Halogenoalkanes H Naming Halogenoalkanes H H H H C H H H H Based on original alkane, with a prefix indicating halogen atom: H C C C H Fluoro for F; Chloro for Cl; Bromo for Br; Iodo for I. H C C C C H Br H H H Cl H H Substituents are listed alphabetically 1-bromopropane 2-chloro-2-methylbutane Classifying Halogenoalkanes Halogenoalkanes can be classified as primary, secondary or tertiary depending on the number of carbon atoms attached to the C-X functional group. H H H H H H H H C H H H H H C C C H H C C C H H C C C C H Br H H H Br H H Cl H H Primary halogenoalkane Secondary halogenoalkane Tertiary halogenoalkane One carbon attached to the Two carbons attached to the Three carbons attached to the carbon atom adjoining the carbon atom adjoining the carbon atom adjoining the halogen halogen halogen Reactions of Halogenoalkanes Halogenoalkanes undergo either substitution or elimination reactions Nucleophilic substitution reactions Substitution: swapping a halogen atom for another atom or groups of atoms - - Nucleophile: electron pair donator e.g. :OH , :NH3, CN :Nu represents any nucleophile – they The Mechanism: We draw (or outline) mechanisms to always have a lone pair and act as show in detail how a reaction proceeds electron pair donators Nu:- The nucleophiles The carbon has a small attack the positive H H H H positive charge because carbon atom of the electronegativity H C C X H C C + X- δ+ δ- Nu difference between the carbon and the halogen H H H H We use curly arrows in mechanisms (with A curly arrow will always two line heads) to show the movement of start from a of electrons or two electrons lone pair the centre of a bond The rate of these substitution reactions depends on the strength Bond enthalpy / of the C-X bond kJmol-1 The weaker the bond, the easier it is to break and the faster the reaction. -
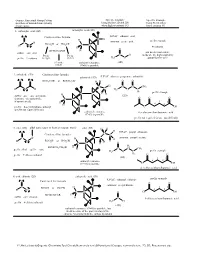
Generic Functional Group Pattern in Ordero of Nomenclature Priority in Our Course. Specific Example Using Suffix (2D and 3D)
Generic Functional Group Pattern Specific Example Specific Example in ordero of nomenclature priority Using Suffix (2D and 3D) Using Prefix when in our course. when highest priority FG lower priority FG 1. carboxylic acid (2D) carboxylic acid (3D) O Condensed line formula IUPAC: ethanoic acid O H H prefix example C H common: acetic acid RCO2H or HO2CR C C R O #-carboxy H O RCH(CO H)R' H suffix: -oic acid 2 O not used in our course FG on FG on C H (acids are the highest priority prefix: #-carboxy the right the left H3C O group that we use) FG in the carbonyl resonance (2D) middle (C=O) is possible 2. anhydride (2D) Condensed line formula anhydride (3D) IUPAC: ethanoic propanoic anhydride O O RCO2COR' or ROCO2CR' H H O O H C C C CH C 3 R O R H3C O C H H 2 prefix example H O C H suffix: -oic -oic anhydride (2D) (just one -oic anhydride, O O O C C O if symmetrical) 3 1 H 4 H 2 prefix: #-acyloxyalkanecarbonyl O O OH (prefix not required for us) carbonyl resonance 4-acyloxymethanebutanoic acid (C=O) is possible (prefix not required for us - too difficult) 3. ester (2D) alkyl name (goes in front as separate word) ester (3D) H H H IUPAC: propyl ethanoate O C Condensed line formula C O C common: propyl acetate C R' H H R O RCO2R' or R'O2CR H H C C H O H H2 RCH(CO2CH3)R' prefix: alkyl suffix: -oate H C C CH3 O H C O C prefix example 3 H prefix: #-alkoxycarbonyl 2 O O (2D) 3 1 carbonyl resonance 4 2 (C=O) is possible O OH 4-methoxycarbonylbutanoic acid 4. -

Chapter 20 the Carbonyl Can Be an Electrophile, It Can Be Attacked. It Can Occur Under Basic Or Acidic Conditions
Chapter 20 The carbonyl can be an electrophile, it can be attacked. It can occur under basic or acidic conditions. Basic Conditions: H O 1. Nu- O 2. H2O workup Nu Nu: HOH O : Nu Acidic Conditions: H+ H O : 1. H+, Nu: O Nu H + O OH Nu Nu: Hydride reductions. Basic conditions, H- is the nucleophile Ketones and aldehydes react w/ LiAlH4. H O 1. LiAlH4 O 2. H2O workup - H H: HOH O : H Esters and acid chlorides react w/ LiAlH4. H O 1. LiAlH4 O 2. H O workup OMe 2 H - H H: HOH O : O O : OMe H H H - H H: There are many sources of hydride with various reactivities Hydride Aldehyde Ketone Ester Amide Carboxyllic Acid Donors acid/ acid salt halide LiAlH4 Alcohol Alcohol Alcohol Amine Alcohol Alcohol LiAlH(OtBu)3 Alcohol Alcohol Alcohol - (too slow) N.R. Aldehyde NaBH4 Alcohol Alcohol N.R. N.R. N.R. ? NaBH3CN Alcohol N.R. N.R. N.R. N.R. ? DIBAL-H Alcohol Alcohol Aldehyde * Aldehyde* Alcohol ? * Stoichiometry must be carefully controlled. H2, Pd/C reacts preferentially with carbon-carbon double bonds. Carbon Nucleophiles: - Both of the following create reagents that act like CH3 . EtBr + 2Li →EtLi + LiBr EtBr + Mg → EtMgBr Alkyne nucleophiles. - + CH3C≡CH + NaNH2→ CH3C≡C: Na + NH3 - + CH3C≡CH + CH3Li → CH3C≡C: Li + CH4↑ CH3C≡CH + CH3MgBr → CH3C≡CMgBr + CH4↑ Ketones and aldehydes react w/ these carbon nucleophiles. H O 1. H3C CCMgBr O 2. H2O workup - C CH3CC: C CH HOH 3 O : Esters and acid chlorides react w/ these carbon nucleophiles. -

Using Carbon Dioxide As a Building Block in Organic Synthesis
REVIEW Received 10 Jul 2014 | Accepted 21 Nov 2014 | Published 20 Jan 2015 DOI: 10.1038/ncomms6933 Using carbon dioxide as a building block in organic synthesis Qiang Liu1, Lipeng Wu1, Ralf Jackstell1 & Matthias Beller1 Carbon dioxide exits in the atmosphere and is produced by the combustion of fossil fuels, the fermentation of sugars and the respiration of all living organisms. An active goal in organic synthesis is to take this carbon—trapped in a waste product—and re-use it to build useful chemicals. Recent advances in organometallic chemistry and catalysis provide effective means for the chemical transformation of CO2 and its incorporation into synthetic organic molecules under mild conditions. Such a use of carbon dioxide as a renewable one-carbon (C1) building block in organic synthesis could contribute to a more sustainable use of resources. more sensible resource management is the prerequisite for the sustainable development of future generations. However, when dealing with the feedstock of the chemical Aindustry, the level of sustainability is still far from satisfactory. Until now, the vast majority of carbon resources are based on crude oil, natural gas and coal. In addition to biomass, CO2 offers the possibility to create a renewable carbon economy. Since pre-industrial times, the amount of CO2 has steadily increased and nowadays CO2 is a component of greenhouse gases, which are primarily responsible for the rise in atmospheric temperature and probably abnormal changes in the global climate. This increase in CO2 concentration is largely due to the combustion of fossil fuels, which are required to meet the world’s energy demand1. -

Nomenclature of Carboxylic Acids • Select the Longest Carbon Chain Containing the Carboxyl Group
Chapter 5 Carboxylic Acids and Esters Carboxylic Acids • Carboxylic acids are weak organic acids which Chapter 5 contain the carboxyl group (RCO2H): Carboxylic Acids and Esters O C O H O RCOOH RCO2H Chapter Objectives: O condensed ways of • Learn to recognize the carboxylic acid, ester, and related functional groups. RCOH writing the carboxyl • Learn the IUPAC system for naming carboxylic acids and esters. group a carboxylic acid C H • Learn the important physical properties of the carboxylic acids and esters. • Learn the major chemical reaction of carboxylic acids and esters, and learn how to O predict the products of ester synthesis and hydrolysis reactions. the carboxyl group • Learn some of the important properties of condensation polymers, especially the polyesters. Mr. Kevin A. Boudreaux • The tart flavor of sour-tasting foods is often caused Angelo State University CHEM 2353 Fundamentals of Organic Chemistry by the presence of carboxylic acids. Organic and Biochemistry for Today (Seager & Slabaugh) www.angelo.edu/faculty/kboudrea 2 Nomenclature of Carboxylic Acids • Select the longest carbon chain containing the carboxyl group. The -e ending of the parent alkane name is replaced by the suffix -oic acid. • The carboxyl carbon is always numbered “1” but the number is not included in the name. • Name the substituents attached to the chain in the Nomenclature of usual way. • Aromatic carboxylic acids (i.e., with a CO2H Carboxylic Acids directly connected to a benzene ring) are named after the parent compound, benzoic acid. O C OH 3 -

Introduction to Alkenes and Alkynes in an Alkane, All Covalent Bonds
Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene, however, only three σ bonds are formed from the alkene carbon -the carbon thus adopts an sp2 hybridization Ethene (common name ethylene) has a molecular formula of CH2CH2 Each carbon is sp2 hybridized with a σ bond to two hydrogens and the other carbon Hybridized orbital allows stronger bonds due to more overlap H H C C H H Structure of Ethylene In addition to the σ framework of ethylene, each carbon has an atomic p orbital not used in hybridization The two p orbitals (each with one electron) overlap to form a π bond (p bonds are not symmetric about the internuclear axis) π bonds are not as strong as σ bonds (in ethylene, the σ bond is ~90 Kcal/mol and the π bond is ~66 Kcal/mol) Thus while σ bonds are stable and very few reactions occur with C-C bonds, π bonds are much more reactive and many reactions occur with C=C π bonds Nomenclature of Alkenes August Wilhelm Hofmann’s attempt for systematic hydrocarbon nomenclature (1866) Attempted to use a systematic name by naming all possible structures with 4 carbons Quartane a alkane C4H10 Quartyl C4H9 Quartene e alkene C4H8 Quartenyl C4H7 Quartine i alkine → alkyne C4H6 Quartinyl C4H5 Quartone o C4H4 Quartonyl C4H3 Quartune u C4H2 Quartunyl C4H1 Wanted to use Quart from the Latin for 4 – this method was not embraced and BUT has remained Used English order of vowels, however, to name the groups -

A Perovskite-Based Paper Microfluidic Sensor for Haloalkane Assays
ORIGINAL RESEARCH published: 26 April 2021 doi: 10.3389/fchem.2021.682006 A Perovskite-Based Paper Microfluidic Sensor for Haloalkane Assays Lili Xie 1, Jie Zan 1, Zhijian Yang 1, Qinxia Wu 1, Xiaofeng Chen 1, Xiangyu Ou 1, Caihou Lin 2*, Qiushui Chen 1,3* and Huanghao Yang 1,3* 1 Ministry of Education (MOE) Key Laboratory for Analytical Science of Food Safety and Biology, Fujian Provincial Key Laboratory of Analysis and Detection Technology for Food Safety, College of Chemistry, Fuzhou University, Fuzhou, China, 2 Department of Neurosurgery, Fujian Medical University Union Hospital, Fuzhou, China, 3 Fujian Science and Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou, China Detection of haloalkanes is of great industrial and scientific importance because some Edited by: haloalkanes are found serious biological and atmospheric issues. The development Jinyi Lin, of a flexible, wearable sensing device for haloalkane assays is highly desired. Here, Nanjing Tech University, China we develop a paper-based microfluidic sensor to achieve low-cost, high-throughput, Reviewed by: Xiaobao Xu, and convenient detection of haloalkanes using perovskite nanocrystals as a nanoprobe Nanjing University of Science and through anion exchanging. We demonstrate that the CsPbX3 (X = Cl, Br, or I) Technology, China nanocrystals are selectively and sensitively in response to haloalkanes (CH2Cl2, CH2Br2), Xuewen Wang, Northwestern Polytechnical and their concentrations can be determined as a function of photoluminescence University, China spectral shifts of perovskite nanocrystals. In particular, an addition of nucleophilic trialkyl *Correspondence: phosphines (TOP) or a UV-photon-induced electron transfer from CsPbX3 nanocrystals is Caihou Lin [email protected] responsible for achieving fast sensing of haloalkanes. -

Reactions of Aromatic Compounds Just Like an Alkene, Benzene Has Clouds of Electrons Above and Below Its Sigma Bond Framework
Reactions of Aromatic Compounds Just like an alkene, benzene has clouds of electrons above and below its sigma bond framework. Although the electrons are in a stable aromatic system, they are still available for reaction with strong electrophiles. This generates a carbocation which is resonance stabilized (but not aromatic). This cation is called a sigma complex because the electrophile is joined to the benzene ring through a new sigma bond. The sigma complex (also called an arenium ion) is not aromatic since it contains an sp3 carbon (which disrupts the required loop of p orbitals). Ch17 Reactions of Aromatic Compounds (landscape).docx Page1 The loss of aromaticity required to form the sigma complex explains the highly endothermic nature of the first step. (That is why we require strong electrophiles for reaction). The sigma complex wishes to regain its aromaticity, and it may do so by either a reversal of the first step (i.e. regenerate the starting material) or by loss of the proton on the sp3 carbon (leading to a substitution product). When a reaction proceeds this way, it is electrophilic aromatic substitution. There are a wide variety of electrophiles that can be introduced into a benzene ring in this way, and so electrophilic aromatic substitution is a very important method for the synthesis of substituted aromatic compounds. Ch17 Reactions of Aromatic Compounds (landscape).docx Page2 Bromination of Benzene Bromination follows the same general mechanism for the electrophilic aromatic substitution (EAS). Bromine itself is not electrophilic enough to react with benzene. But the addition of a strong Lewis acid (electron pair acceptor), such as FeBr3, catalyses the reaction, and leads to the substitution product. -

Linear Alkylbenzene Sulphonate (CAS No
Environmental Risk Assessment LAS Linear Alkylbenzene Sulphonate (CAS No. 68411-30-3) Revised ENVIRONMENTAL Aspect of the HERA Report = February 2013 = 1 1. Contents 2. Executive summary 3. Substance characterisation 3.1 CAS No. and grouping information 3.2 Chemical structure and composition 3.3 Manufacturing route and production/volume statistics 3.4 Consumption scenario in Europe 3.5 Use application summary 4. Environmental safety assessment 4.1 Environmental exposure assessment 4.1.1 Biotic and abiotic degradability 4.1.2 Removal 4.1.3 Monitoring studies 4.1.4 Exposure assessment: scenario description 4.1.5 Substance data used for the exposure calculation 4.1.6 PEC calculations 4.1.7 Bioconcentration 4.2 Environmental effects assessment 4.2.1 Ecotoxicity 4.2.1.1 Aquatic ecotoxicity 4.2.1.2 Terrestrial ecotoxicity 4.2.1.3 Sediment ecotoxicity 4.2.1.4 Ecotoxicity to sewage microorganisms 4.2.1.5 Reassurance on absence of estrogenic effects 4.2.2 PNEC calculations 4.2.2.1 Aquatic PNEC 4.2.2.2 Terrestrial PNEC 4.2.2.3 Sludge PNEC 4.2.2.4 Sediment PNEC 4.2.2.5 STP PNEC 4.3 Environment risk assessment 5. 5. References 6. Contributors to the report 6.1 Substance team 6.2 HERA environmental task force 6.3 HERA human health task force 6.4 Industry coalition for the OECD/ICCA SIDS assessment of LAS 2 2. Executive Summary Linear alkylbenzene sulphonate (LAS) is an anionic surfactant. It was introduced in 1964 as the readily biodegradable replacement for highly branched alkylbenzene sulphonates (ABS).