Chapter 20 the Carbonyl Can Be an Electrophile, It Can Be Attacked. It Can Occur Under Basic Or Acidic Conditions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Products from Reactions of Carbon Nucleophiles and Carbon
14C synthesis strategies, Chem 315/316 / Beauchamp 1 Products from reactions of carbon nucleophiles and carbon electrophiles used in the 14C Game and our course: Carbon and hydrogen nucleophiles Ph R Ph Al H N R Li R (MgBr) P Li AlH R Cu Li Na CN RCC Na Ph 4 Li Carbon 2 H C R organolithium organolithium cyanide acetylides 2 ylid Na BH4 diisobutylaluminium lithium diisopropy- electrophiles cuprates (LAH) o reagents reagents Wittig reagents hydride (DIBAH) amide (LDA), -78 C H C Br 3 not useful not useful 2 RX coupling nitrilesalkynes not useful alkyls alkyls not useful methyl RX reaction R Br not useful not useful 2 RX coupling nitriles alkynes not useful alkyls alkyls not useful primary RX reaction R 2 RX coupling nitriles alkyls not useful E2 not useful alkyls not useful not useful reaction R Br secondary RX O 1o ROH 1o ROH 1o ROH 1o ROH not useful 1o ROH o not useful not useful 1o ROH 1 ROH ethylene oxide nitriles alkynes O o o o o o o 2 ROH 2 ROH 2 ROH 2 ROH 2o ROH 2 ROH 2 ROH not useful not useful E2, make nitriles alkynes allylic alcohols propylene oxide O o 3 ROH 3o ROH o 3o ROH o not useful o 3o ROH not useful E2, make 3 ROH 3 ROH 3 ROH allylic alcohols nitriles alkynes isobutylene oxide O o o specific methanol methanol C 1 ROH 1 ROH not used cyanohydrin 1o ROH not useful not useful in our course alkenes H H alkynes methanal O specific o enolate o o cyanohydrin o 1 ROH o C 2 ROH 2 ROH not useful 2 ROH alkenes 1 ROH not useful chemistry R H alkynes simple aldehydes O cyanohydrin o unless specific o enolate C 3 ROH 3o ROH o 2o ROH -

Chapter 7, Haloalkanes, Properties and Substitution Reactions of Haloalkanes Table of Contents 1
Chapter 7, Haloalkanes, Properties and Substitution Reactions of Haloalkanes Table of Contents 1. Alkyl Halides (Haloalkane) 2. Nucleophilic Substitution Reactions (SNX, X=1 or 2) 3. Nucleophiles (Acid-Base Chemistry, pka) 4. Leaving Groups (Acid-Base Chemistry, pka) 5. Kinetics of a Nucleophilic Substitution Reaction: An SN2 Reaction 6. A Mechanism for the SN2 Reaction 7. The Stereochemistry of SN2 Reactions 8. A Mechanism for the SN1 Reaction 9. Carbocations, SN1, E1 10. Stereochemistry of SN1 Reactions 11. Factors Affecting the Rates of SN1 and SN2 Reactions 12. --Eliminations, E1 and E2 13. E2 and E1 mechanisms and product predictions In this chapter we will consider: What groups can be replaced (i.e., substituted) or eliminated The various mechanisms by which such processes occur The conditions that can promote such reactions Alkyl Halides (Haloalkane) An alkyl halide has a halogen atom bonded to an sp3-hybridized (tetrahedral) carbon atom The carbon–chlorine and carbon– bromine bonds are polarized because the halogen is more electronegative than carbon The carbon-iodine bond do not have a per- manent dipole, the bond is easily polarizable Iodine is a good leaving group due to its polarizability, i.e. its ability to stabilize a charge due to its large atomic size Generally, a carbon-halogen bond is polar with a partial positive () charge on the carbon and partial negative () charge on the halogen C X X = Cl, Br, I Different Types of Organic Halides Alkyl halides (haloalkanes) sp3-hybridized Attached to Attached to Attached -

Reactions of Benzene & Its Derivatives
Organic Lecture Series ReactionsReactions ofof BenzeneBenzene && ItsIts DerivativesDerivatives Chapter 22 1 Organic Lecture Series Reactions of Benzene The most characteristic reaction of aromatic compounds is substitution at a ring carbon: Halogenation: FeCl3 H + Cl2 Cl + HCl Chlorobenzene Nitration: H2 SO4 HNO+ HNO3 2 + H2 O Nitrobenzene 2 Organic Lecture Series Reactions of Benzene Sulfonation: H 2 SO4 HSO+ SO3 3 H Benzenesulfonic acid Alkylation: AlX3 H + RX R + HX An alkylbenzene Acylation: O O AlX H + RCX 3 CR + HX An acylbenzene 3 Organic Lecture Series Carbon-Carbon Bond Formations: R RCl AlCl3 Arenes Alkylbenzenes 4 Organic Lecture Series Electrophilic Aromatic Substitution • Electrophilic aromatic substitution: a reaction in which a hydrogen atom of an aromatic ring is replaced by an electrophile H E + + + E + H • In this section: – several common types of electrophiles – how each is generated – the mechanism by which each replaces hydrogen 5 Organic Lecture Series EAS: General Mechanism • A general mechanism slow, rate + determining H Step 1: H + E+ E El e ctro - Resonance-stabilized phile cation intermediate + H fast Step 2: E + H+ E • Key question: What is the electrophile and how is it generated? 6 Organic Lecture Series + + 7 Organic Lecture Series Chlorination Step 1: formation of a chloronium ion Cl Cl + + - - Cl Cl+ Fe Cl Cl Cl Fe Cl Cl Fe Cl4 Cl Cl Chlorine Ferric chloride A molecular complex An ion pair (a Lewis (a Lewis with a positive charge containing a base) acid) on ch lorine ch loronium ion Step 2: attack of -

Using Carbon Dioxide As a Building Block in Organic Synthesis
REVIEW Received 10 Jul 2014 | Accepted 21 Nov 2014 | Published 20 Jan 2015 DOI: 10.1038/ncomms6933 Using carbon dioxide as a building block in organic synthesis Qiang Liu1, Lipeng Wu1, Ralf Jackstell1 & Matthias Beller1 Carbon dioxide exits in the atmosphere and is produced by the combustion of fossil fuels, the fermentation of sugars and the respiration of all living organisms. An active goal in organic synthesis is to take this carbon—trapped in a waste product—and re-use it to build useful chemicals. Recent advances in organometallic chemistry and catalysis provide effective means for the chemical transformation of CO2 and its incorporation into synthetic organic molecules under mild conditions. Such a use of carbon dioxide as a renewable one-carbon (C1) building block in organic synthesis could contribute to a more sustainable use of resources. more sensible resource management is the prerequisite for the sustainable development of future generations. However, when dealing with the feedstock of the chemical Aindustry, the level of sustainability is still far from satisfactory. Until now, the vast majority of carbon resources are based on crude oil, natural gas and coal. In addition to biomass, CO2 offers the possibility to create a renewable carbon economy. Since pre-industrial times, the amount of CO2 has steadily increased and nowadays CO2 is a component of greenhouse gases, which are primarily responsible for the rise in atmospheric temperature and probably abnormal changes in the global climate. This increase in CO2 concentration is largely due to the combustion of fossil fuels, which are required to meet the world’s energy demand1. -

Reactions of Aromatic Compounds Just Like an Alkene, Benzene Has Clouds of Electrons Above and Below Its Sigma Bond Framework
Reactions of Aromatic Compounds Just like an alkene, benzene has clouds of electrons above and below its sigma bond framework. Although the electrons are in a stable aromatic system, they are still available for reaction with strong electrophiles. This generates a carbocation which is resonance stabilized (but not aromatic). This cation is called a sigma complex because the electrophile is joined to the benzene ring through a new sigma bond. The sigma complex (also called an arenium ion) is not aromatic since it contains an sp3 carbon (which disrupts the required loop of p orbitals). Ch17 Reactions of Aromatic Compounds (landscape).docx Page1 The loss of aromaticity required to form the sigma complex explains the highly endothermic nature of the first step. (That is why we require strong electrophiles for reaction). The sigma complex wishes to regain its aromaticity, and it may do so by either a reversal of the first step (i.e. regenerate the starting material) or by loss of the proton on the sp3 carbon (leading to a substitution product). When a reaction proceeds this way, it is electrophilic aromatic substitution. There are a wide variety of electrophiles that can be introduced into a benzene ring in this way, and so electrophilic aromatic substitution is a very important method for the synthesis of substituted aromatic compounds. Ch17 Reactions of Aromatic Compounds (landscape).docx Page2 Bromination of Benzene Bromination follows the same general mechanism for the electrophilic aromatic substitution (EAS). Bromine itself is not electrophilic enough to react with benzene. But the addition of a strong Lewis acid (electron pair acceptor), such as FeBr3, catalyses the reaction, and leads to the substitution product. -

Reactions of Alkenes and Alkynes
05 Reactions of Alkenes and Alkynes Polyethylene is the most widely used plastic, making up items such as packing foam, plastic bottles, and plastic utensils (top: © Jon Larson/iStockphoto; middle: GNL Media/Digital Vision/Getty Images, Inc.; bottom: © Lakhesis/iStockphoto). Inset: A model of ethylene. KEY QUESTIONS 5.1 What Are the Characteristic Reactions of Alkenes? 5.8 How Can Alkynes Be Reduced to Alkenes and 5.2 What Is a Reaction Mechanism? Alkanes? 5.3 What Are the Mechanisms of Electrophilic Additions HOW TO to Alkenes? 5.1 How to Draw Mechanisms 5.4 What Are Carbocation Rearrangements? 5.5 What Is Hydroboration–Oxidation of an Alkene? CHEMICAL CONNECTIONS 5.6 How Can an Alkene Be Reduced to an Alkane? 5A Catalytic Cracking and the Importance of Alkenes 5.7 How Can an Acetylide Anion Be Used to Create a New Carbon–Carbon Bond? IN THIS CHAPTER, we begin our systematic study of organic reactions and their mecha- nisms. Reaction mechanisms are step-by-step descriptions of how reactions proceed and are one of the most important unifying concepts in organic chemistry. We use the reactions of alkenes as the vehicle to introduce this concept. 129 130 CHAPTER 5 Reactions of Alkenes and Alkynes 5.1 What Are the Characteristic Reactions of Alkenes? The most characteristic reaction of alkenes is addition to the carbon–carbon double bond in such a way that the pi bond is broken and, in its place, sigma bonds are formed to two new atoms or groups of atoms. Several examples of reactions at the carbon–carbon double bond are shown in Table 5.1, along with the descriptive name(s) associated with each. -

10: Alkenes and Alkynes. Electrophilic and Concerted Addition Reactions
(2/94)(8,9/96)(12/03)(1,2/04) Neuman Chapter 10 10: Alkenes and Alkynes. Electrophilic and Concerted Addition Reactions Addition Reactions Electrophilic Addition of H-X or X2 to Alkenes Addition of H-X and X2 to Alkynes Alkenes to Alcohols by Electrophilic Addition Alkenes to Alcohols by Hydroboration Addition of H2 to Alkenes and Alkynes Preview (To be added) 10.1 Addition Reactions We learned about elimination reactions that form C=C and Cº C bonds in Chapter 9. In this chapter we learn about reactions in which reagents add to these multiple bonds. General Considerations (10.1A) We show a general equation for an addition reaction with an alkene in Figure 10.01. Figure 10.01 (see Figure folder for complete figure) A¾ B A B | | C=C ® C¾ C This equation is the reverse of the general equation for an elimination reaction that we showed at the beginning of Chapter 9. This general equation does not show a mechanism for the addition process. There are a number of different types of mechanisms for addition reactions, but we can group them into the four broad categories of (1) electrophilic addition, (2) nucleophilic addition, (3) free radical addition, and (4) concerted addition. Electrophilic and nucleophilic addition reactions involve intermediate ions so they are ionic addition reactions. In contrast, free radical additions, and 1 (2/94)(8,9/96)(12/03)(1,2/04) Neuman Chapter 10 concerted addition reactions, are non-ionic addition reactions because they do not involve the formation of intermediate ions. Ionic Addition Reactions (10.1B) We compare general features of nucleophilic and electrophilic addition reactions here. -

Chapter 16 the Chemistry of Benzene and Its Derivatives
Instructor Supplemental Solutions to Problems © 2010 Roberts and Company Publishers Chapter 16 The Chemistry of Benzene and Its Derivatives Solutions to In-Text Problems 16.1 (b) o-Diethylbenzene or 1,2-diethylbenzene (d) 2,4-Dichlorophenol (f) Benzylbenzene or (phenylmethyl)benzene (also commonly called diphenylmethane) 16.2 (b) (d) (f) (h) 16.3 Add about 25 °C per carbon relative to toluene (110.6 C; see text p. 743): (b) propylbenzene: 161 °C (actual: 159 °C) 16.4 The aromatic compound has NMR absorptions with greater chemical shift in each case because of the ring current (Fig. 16.2, text p. 745). (b) The chemical shift of the benzene protons is at considerably greater chemical shift because benzene is aromatic and 1,4-cyclohexadiene is not. 16.6 (b) Among other features, the NMR spectrum of 1-bromo-4-ethylbenzene has a typical ethyl quartet and a typical para-substitution pattern for the ring protons, as shown in Fig. 16.3, text p. 747, whereas the spectrum of (2- bromoethyl)benzene should show a pair of triplets for the methylene protons and a complex pattern for the ring protons. If this isn’t enough to distinguish the two compounds, the integral of the ring protons relative to the integral of the remaining protons is different in the two compounds. 16.7 (b) The IR spectrum indicates the presence of an OH group, and the chemical shift of the broad NMR resonance (d 6.0) suggests that this could be a phenol. The splitting patterns of the d 1.17 and d 2.58 resonances show that the compound also contains an ethyl group, and the splitting pattern of the ring protons shows that the compound is a para-disubstituted benzene derivative. -

Reactions of Alkenes Since Bonds Are Stronger Than Bonds, Double Bonds Tend to React to Convert the Double Bond Into Bonds
Reactions of Alkenes Since bonds are stronger than bonds, double bonds tend to react to convert the double bond into bonds This is an addition reaction. (Other types of reaction have been substitution and elimination). Addition reactions are typically exothermic. Electrophilic Addition The bond is localized above and below the C-C bond. The electrons are relatively far away from the nuclei and are therefore loosely bound. An electrophile will attract those electrons, and can pull them away to form a new bond. This leaves one carbon with only 3 bonds and a +ve charge (carbocation). The double bond acts as a nucleophile (attacks the electrophile). Ch08 Reacns of Alkenes (landscape) Page 1 In most cases, the cation produced will react with another nucleophile to produce the final overall electrophilic addition product. Electrophilic addition is probably the most common reaction of alkenes. Consider the electrophilic addition of H-Br to but-2-ene: The alkene abstracts a proton from the HBr, and a carbocation and bromide ion are generated. The bromide ion quickly attacks the cationic center and yields the final product. In the final product, H-Br has been added across the double bond. Ch08 Reacns of Alkenes (landscape) Page 2 Orientation of Addition Consider the addition of H-Br to 2-methylbut-2-ene: There are two possible products arising from the two different ways of adding H-Br across the double bond. But only one is observed. The observed product is the one resulting from the more stable carbocation intermediate. Tertiary carbocations are more stable than secondary. -

Electrophilic Addition Reactions.Pdf
Electrophilic Addition Reactions Electrophilic addition reactions are an important class of reactions that allow the interconversion of C=C and C≡C into a range of important functional groups. Conceptually, addition is the reverse of elimination What does the term "electrophilic addition" imply ? A electrophile, E+, is an electron poor species that will react with an electron rich species (the C=C) An addition implies that two systems combine to a single entity. Depending on the relative timing of these events, slightly different mechanisms are possible: • Reaction of the C=C with E+ to give a carbocation (or another cationic intermediate) that then reacts with a Nu- • Simultaneous formation of the two σ bonds The following pointers may aid your understanding of these reactions: • Recognise the electrophile present in the reagent combination • The electrophile adds first to the alkene, dictating the regioselectivity. • If the reaction proceeds via a planar carbocation, the reaction is not stereoselective • If the two new σ bonds form at the same time from the same species, then syn addition is observed • If the two new σ bonds form at different times from different species, then anti addition is observed Hydrogenation of Alkenes Reaction Type: Electrophilic Addition Summary • Alkenes can be reduced to alkanes with H2 in the presence of metal catalysts such as Pt, Pd, Ni or Rh. • The two new C-H σ bonds are formed simultaneously from H atoms absorbed into the metal surface. • The reaction is stereospecific giving only the syn addition product. • This reaction forms the basis of experimental "heats of hydrogenation" which can be used to establish the stability of isomeric alkenes. -
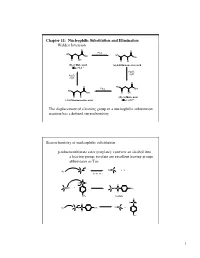
Chapter 11: Nucleophilic Substitution and Elimination Walden Inversion
Chapter 11: Nucleophilic Substitution and Elimination Walden Inversion O O PCl5 HO HO OH OH O OH O Cl (S)-(-) Malic acid (+)-2-Chlorosuccinic acid [a]D= -2.3 ° Ag2O, H2O Ag2O, H2O O O HO PCl5 OH HO OH O OH O Cl (R)-(+) Malic acid (-)-2-Chlorosuccinic acid [a]D= +2.3 ° The displacement of a leaving group in a nucleophilic substitution reaction has a defined stereochemistry Stereochemistry of nucleophilic substitution p-toluenesulfonate ester (tosylate): converts an alcohol into a leaving group; tosylate are excellent leaving groups. abbreviates as Tos C X Nu C + X- Nu: X= Cl, Br, I O Cl S O O + C OH C O S CH3 O CH3 tosylate O -O S O O Nu C + Nu: C O S CH3 O CH3 1 O Tos-Cl - H3C O O H + TosO - H O H pyridine H O Tos O CH3 [a]D= +33.0 [a]D= +31.1 [a]D= -7.06 HO- HO- O - Tos-Cl H3C O - H O O TosO + H O H pyridine Tos H O CH3 [a]D= -7.0 [a]D= -31.0 [a]D= -33.2 The nucleophilic substitution reaction “inverts” the Stereochemistry of the carbon (electrophile)- Walden inversion Kinetics of nucleophilic substitution Reaction rate: how fast (or slow) reactants are converted into product (kinetics) Reaction rates are dependent upon the concentration of the reactants. (reactions rely on molecular collisions) H H Consider: HO C _ _ C Br Br HO H H H H At a given temperature: If [OH-] is doubled, then the reaction rate may be doubled If [CH3-Br] is doubled, then the reaction rate may be doubled A linear dependence of rate on the concentration of two reactants is called a second-order reaction (molecularity) 2 H H HO C _ _ C Br Br HO H H H H Reaction rates (kinetic) can be expressed mathematically: reaction rate = disappearance of reactants (or appearance of products) For the disappearance of reactants: - rate = k [CH3Br] [OH ] [CH3Br] = CH3Br concentration [OH-] = OH- concentration k= constant (rate constant) L mol•sec For the reaction above, product formation involves a collision between both reactants, thus the rate of the reaction is dependent upon the concentration of both. -

Chemistry Education Research and Practice Accepted Manuscript
Chemistry Education Research and Practice Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. We will replace this Accepted Manuscript with the edited and formatted Advance Article as soon as it is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/cerp Page 1 of 23 Chemistry Education Research and Practice 1 2 3 4 Organic Chemistry Students’ Ideas about Nucleophiles and 5 6 Electrophiles: The Role of Charges and Mechanisms 7 8 9 Mary E. Anzovino and Stacey Lowery Bretz* 10 11 Miami University, Department of Chemistry & Biochemistry, Oxford, OH USA. Email: [email protected] 12 13 Organic chemistry students struggle with reaction mechanisms and the electron-pushing formalism (EPF) used by Manuscript 14 15 practicing organic chemists. Faculty have identified an understanding of nucleophiles and electrophiles as one conceptual 16 prerequisite to mastery of the EPF, but little is known about organic chemistry students’ knowledge of nucleophiles and 17 electrophiles.