United States Patent Office Patented Nov
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Synthesis of Novel Single-Source Precursors for CVD of Mixed-Metal Tungsten Oxide
Synthesis of novel single-source precursors for CVD of mixed-metal tungsten oxide Hamid Choujaa A thesis submitted for the degree of Doctor of Philosophy University of Bath Department of Chemistry March 2008 COPYRIGHT Attention is drawn to the fact that copyright of this thesis rests with its author. This copy of the thesis has been supplied on condition that anyone who consults it is understood to recognize that its copyright rests with its author and that no quotation the thesis and no information derived from it may be published without the prior written consent of the author. This thesis may be made available for consultation within the University Library and may be photocopied or lent to other libraries for the purposes of consultation. TABLE OF CONTENTS Abstract ....................................................................................................................................... i Acknowledgements .................................................................................................................... iii Abbreviations and Acronyms .................................................................................................... iv 1. INTRODUCTION .................................................................................................................. 1 1.1 Generality about tungsten(VI) oxide ............................................................................. 1 1.1.1 The different lattice structures of tungsten oxide ........................................... 1 1.1.2 Electronic and -

8.6 Acidity of Alcohols and Thiols 355
08_BRCLoudon_pgs5-1.qxd 12/8/08 11:05 AM Page 355 8.6 ACIDITY OF ALCOHOLS AND THIOLS 355 ural barrier to the passage of ions. However, the hydrocarbon surface of nonactin allows it to enter readily into, and pass through, membranes. Because nonactin binds and thus transports ions, the ion balance crucial to proper cell function is upset, and the cell dies. Ion Channels Ion channels, or “ion gates,” provide passageways for ions into and out of cells. (Recall that ions are not soluble in membrane phospholipids.) The flow of ions is essen- tial for the transmission of nerve impulses and for other biological processes. A typical chan- nel is a large protein molecule imbedded in a cell membrane. Through various mechanisms, ion channels can be opened or closed to regulate the concentration of ions in the interior of the cell. Ions do not diffuse passively through an open channel; rather, an open channel contains regions that bind a specific ion. Such an ion is bound specifically within the channel at one side of the membrane and is somehow expelled from the channel on the other side. Remark- ably, the structures of the ion-binding regions of these channels have much in common with the structures of ionophores such as nonactin. The first X-ray crystal structure of a potassium- ion channel was determined in 1998 by a team of scientists at Rockefeller University led by Prof. Roderick MacKinnon (b. 1956), who shared the 2003 Nobel Prize in Chemistry for this work. The interior of the channel contains binding sites for two potassium ions; these sites are oxygen-rich, much like the interior of nonactin. -

Nitric Oxide Activation Facilitated by Cooperative Multimetallic Electron Transfer Within an Iron- Cite This: Chem
Chemical Science View Article Online EDGE ARTICLE View Journal | View Issue Nitric oxide activation facilitated by cooperative multimetallic electron transfer within an iron- Cite this: Chem. Sci.,2018,9,6379 – † All publication charges for this article functionalized polyoxovanadate alkoxide cluster have been paid for by the Royal Society a a a a b of Chemistry F. Li, R. L. Meyer, S. H. Carpenter, L. E. VanGelder, A. W. Nichols, C. W. Machan, b M. L. Neidiga and E. M. Matson *a A series of NO-bound, iron-functionalized polyoxovanadate–alkoxide (FePOV–alkoxide) clusters have been synthesized, providing insight into the role of multimetallic constructs in the coordination and activation of a substrate. Upon exposure of the heterometallic cluster to NO, the vanadium-oxide metalloligand is oxidized by a single electron, shuttling the reducing equivalent to the {FeNO} subunit to 7 V IV form a {FeNO} species. Four NO-bound clusters with electronic distributions ranging from [V3V2 ] Received 1st March 2018 {FeNO}7 to [VIV]{FeNO}7 have been synthesized, and characterized via 1H NMR, infrared, and electronic Accepted 30th June 2018 5 absorption spectroscopies. The ability of the FePOV–alkoxide cluster to store reducing equivalents in the DOI: 10.1039/c8sc00987b Creative Commons Attribution 3.0 Unported Licence. metalloligand for substrate coordination and activation highlights the ultility of the metal-oxide scaffold rsc.li/chemical-science as a redox reservoir. Introduction The activation of NO requires the simultaneous transfer of multiple electrons and protons to the substrate. In nature, The chemical reactivity of nitric oxide (NO) has captivated the eld similar chemical transformations of gaseous substrates (e.g. -

Direct Synthesis of Some Significant Metal Alkoxides
SD0000032 DIRECT SYNTHESIS OF SOME SIGNIFICANT METAL ALKOXI'DE BVYU EMI1JG A THESIS SUBMITTED FOR THE DEGREE OF Mi.Sc. IN CHEMISTRY SUPERVISOR: Dr. O.Y.OMER DEPARTMENT OF CHEMISTRY FACULTY OF EDUCATION UNIVERSITY OF KHARTOUM NOVEMBER, 1998 31/28 DISCLAIMER Portions of this document may be illegible in electronic image products. Images are produced from the best available original document. Please be aware that all of the Missing Pages in this document were originally blank pages Dedication To my three children: Regina, Maria and Samuel CONTENTS Page Dedication i Contents ii List of Tables v List of Figures vii Acknowledgement viii Abstract (Arabic) ix Abstract (English) x CHAPTER 1.0 CHAPTER ONE - INTRODUCTION 1 2.0 CHAPTER TWO - LITERATURE REVIEW 5 2.1 Introduction to Literature Review 5 2.2 Definition of metal alkoxides 5 2.3 Metal elements and (heir chemistry 8 2.3.1 Sodium metal 8 2.3.2 Magnesium metal 12 2.3.3 Aluminium metal 16 2.3.3.1 Hydrolysis of aluminium compounds 20 2.3.4 Tin metal 21 2.4 Preparative methods and uses ofalkoxides ofNa, Mg, Al & Sn. 25 2.4.1 Sodium alkoxide 25 2.4.2 Mamiesium alkoxide 26 2.4.3 Aluminium alkoxide 27 2.4.4. Tin alkoxide 30 2.5 General properties of metal alkoxides 31 2.5.1 1 lydrolysis in metal alkoxide 34 3.0 CHAPTER THREE - MATERIALS AND EXPERIMENTAL PROCEDURE 36 3.1 General procedures 36 3.1.1 Start ing material s 3 6 3.1. I.I Apparatus 36 3.1.1.2 Dry ethanol and isopropanol 36 3.1.1.3 Na, Mg, Al & Sn metals 36 3.1.2 Infrared spectra (Ir) 37 3.2 Reactions procedures 37 3.2.1 Reaction between sodium metal and absolute ethanol 37 3.2.2 Reaction of magnesium metal with absolute ethanol 37 3.2.3 Reaction of magnesium mclal with absolute ethanol using mercury (11) chloride catalyst. -

United States Patent Office Patented May 7, 1963
3,088,959 United States Patent Office Patented May 7, 1963 1. 2 or grouping of carbon atoms which is present in cyclo 3,088,959 pentadiene. This grouping is illustrated as PROCESS OF MAKENG CYCLOPENTADEENY NECKEL, NTROSYL COMPOUNDS Robert D. Feltham, Joseph F. Anzenberger, azad Jonatian T. Carrie, Pittsburgh, Pa., assignors to The Interaa tional Nickel Company, Inc., New York, N.Y., a corpo ration of Delaware No Drawing. FiRed Sept. 1, 1960, Ser. No. 53,374 The substituent groups on the cyclopentadiene moiety 6 Clains. (C. 260-439) 0. indicated as R, R2, R3, R and R5 are any one or more The present invention relates to the production of of hydrogen atoms, halogen atoms and/or organic groups nickel compounds and, more particularly, to the produc such as aliphatic groups, aromatic groups, alicyclic groups, tion of nickel nitrosyl compounds containing a group etc. The substituent groups can also bond at two posi having the cyclopentadienyl moiety. tions. Where this occurs, groups can substitute for adja Compounds such as cyclopentadienylnickel nitrosyl, 5 cent R groups, e.g., Ra and R3 and/or R4 and R5 to form methylcyclopentadienylnickel nitrosyl and other complex indene and other condensed ring structures. nitrosyl compounds containing a cyclopentadienyl-type As mentioned hereinbefore, when carrying out the proc group have been made. Such compounds have use as ess of the present invention, the reactants are reacted in gasoline additives. When such use is contemplated, it is the presence of a base. The base can advantageously be economically imperative that the compounds be produced 20 a nitrogen base or a phosphorus base or an alkoxide of a in good yield from the most readily available and inex metal having a strong hydroxide. -
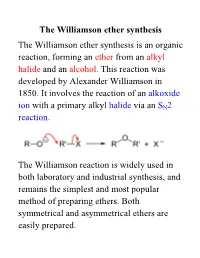
Williamson Ether Synthesis the Williamson Ether Synthesis Is an Organic Reaction, Forming an Ether from an Alkyl Halide and an Alcohol
The Williamson ether synthesis The Williamson ether synthesis is an organic reaction, forming an ether from an alkyl halide and an alcohol. This reaction was developed by Alexander Williamson in 1850. It involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction. The Williamson reaction is widely used in both laboratory and industrial synthesis, and remains the simplest and most popular method of preparing ethers. Both symmetrical and asymmetrical ethers are easily prepared. The reaction for this week: an example of a Williamson ether synthesis acetaminophen ethyl iodide phenacetin starting material reagent product Phenacetin may be synthesized as an example of the Williamson ether synthesis The first synthesis of phenacetin was reported in 1878 by Harmon Morse. Procedure 1. Weigh an Extra-Strength Tylenol tablet. Pulverize the tablet with mortar and pestle. Weigh out 0.22 g and place it in a dry 15-ml round-bottom flask along with 0.28 g of finely pulverized K2CO3 (mortar and pestle) and 3.0 mL of butanone. Carefully add 0.28 mL of ethyl iodide with a syringe. 2. Add a stir bar; attach a microscale water-cooled condenser to the flask. Heat the mixture under reflux directly on a hot plate at medium setting for 1 hour. In the meantime, obtain the IR of acetaminophen. 3. Turn off the heat. Allow the mixture to cool down. Add 4 mL of water to the flask and transfer its contents to a 16 x 125 mm test tube with a screw cap. Rinse round-bottom flask 4 times with 1 mL of tert-butyl methyl ether (BME) and add the rinsings to the test tube. -

United States Patent Office Patented Mar
3,239,568 United States Patent Office Patented Mar. 8, 1966 2 the metal atom bonded to the oxygen atom of the alkoxide 3,239,568 molecule. MANUFACTURE OF ALKALMETAL (COAEPOUNDS Thus the alkali metallometallic alkoxides of this inven David O. De Pree and anaes D. Johnston, Baton Rotage, tion are bifunctional compounds-that is they contain two La., assignors to Ethyl Corporation, New York, N.Y., 5 reactive centers. One of the striking features of this in a corporation of Virginia vention is that in a reaction with a hydrocarbon halide or No Drawing. Filled Aug. 9, 1960, Ser. No. 48,356 hydrocarbon sulfate alkylating agent only the “metal 6 Claims. (C. 260-632) lo,' i.e. This invention involves a process for the manufacture O of organic compounds, in particular, alkali metal alkox --M ides, which can easily be converted to the corresponding functional group reacts, and the "metallic,” i.e. alcohols. This invention is also concerned with bimetal lic organometallic compounds which are utilized in pre paring the alkali metal alkoxides of this invention. --OM Prior to this invention no satisfactory method was reactive center does not. known for increasing the molecular weight of metal alk Although the terms alkali metallometallic alkoxide and oxides using direct reactions of organic halides or organic metal alkoxide are employed throughout this specifica Sulfates. A process which employs hydrocarbon halides tion the process of this invention can be carried out using or hydrocarbon sulfates, and in particular, long chain hy 20 the corresponding alkenoxides and aryioxides, as these drocarbon halides, to produce alkali metal alkoxides of compounds are essentially equivalent to alkoxides for the increased chain length would be of particular value to purposes of this invention. -

Phosphine-Catalyzed Additions of Nucleophiles and Electrophiles to Α
PHOSPHINE-CATALYZED ADDITIONS OF NUCLEOPHILES AND ELECTROPHILES TO α,β–UNSATURATED CARBONYL COMPOUNDS Reported by Michael Scott Bultman November 4, 2004 INTRODUCTION Organophosphorous compounds are becoming increasingly important in organic synthesis. Phosphines serve as precursors to phosphonium ylides in the Wittig reaction,1 and as nucleophilic triggers in the Mitsunobu2 and Staudinger3 reactions. In these processes, the phosphine is stoichiometrically consumed and converted into a phosphine oxide. Phosphines are also commonly used as ligands for transition metal-catalyzed reactions, to modulate reactivity and stereocontrol.4 On the other hand, the use of phosphines as nucleophilic catalysts for organic reactions has only gained attention in the last ten years. First reported by Rauhut and Currier in 1963,5 phosphine catalysis has since been reinvestigated after the phosphine ligands in some transition-metal-catalyzed reactions were found to be better catalysts than the metal/phosphine complexes alone!6 Phosphines are well suited for catalyzing the addition of both nucleophiles and electrophiles to electron deficient alkenes, alkynes, and allenes. Activation of these α,β-unsaturated carbonyl systems with the phosphine enables the formation of new bonds at the α-, β-, and γ-positions. This report will highlight these different modes of addition to α,β-unsaturated carbonyl systems under phosphine catalysis that allow for the formation of a wide array of products from a single class of substrates. GENERAL REACTIVITY OF PHOSPHINES Key characteristics required for successful nucleophilic catalysis lie in the balance of leaving group ability, nucleophilicity, and ease of ylid formation. Increasing leaving group ability can often be + correlated with decreasing basicity. -

Chapter 18 Ethers and Epoxides; Thiols and Sulfides Ethers
Chapter 18 Ethers and Epoxides; Thiols and Sulfides Ethers • Ethers (R–O–R’): – Organic derivatives of water, having two organic groups bonded to the same oxygen atom © 2016 Cengage Learning 2 NAMES AND PROPERTIES OF ETHERS 3 Nomenclature: Common Names • Simple ethers are named by identifying two organic substituents and adding the word ether – Name the groups in alphabetical order – Symmetrical: Use dialkyl or just alkyl © 2016 Cengage Learning 4 Nomenclature: IUPAC Names • The more complex alkyl group is the parent name • The group with the oxygen becomes an alkoxy group © 2016 Cengage Learning 5 Nomenclature: Cyclic Ethers (Heterocycles) • Heterocyclic: Oxygen is part of the ring. O • Epoxides (oxiranes) H2C CH2 O • Oxetanes • Furans (Oxolanes) O O • Pyrans (Oxanes) O O O • Dioxanes O © 2013 Pearson Education, Inc. 6 Epoxide Nomenclature • Name the starting alkene and add “oxide” © 2013 Pearson Education, Inc. 7 Epoxide Nomenclature • The oxygen can be treated as a substituent (epoxy) on the compound • Use numbers to specify position • Oxygen is 1, the carbons are 2 and 3 • Substituents are named in alphabetical order © 2013 Pearson Education, Inc. 8 Properties of Ethers • Possess nearly the same geometry as water – Oxygen atom is sp3-hybridized – Bond angles of R–O–R bonds are approximately tetrahedral • Polar C—O bonds © 2013 Pearson Education, Inc. 9 Properties of Ethers: Hydrogen Bond • Hydrogen bond is a attractive interaction between an electronegative atom and a hydrogen atom bonded to another electronegative atom • Ethers cannot hydrogen bond with other ether molecules, so they have a lower boiling point than alcohols • Ether molecules can hydrogen bond with water and alcohol molecules • They are hydrogen bond acceptors © 2013 Pearson Education, Inc. -

Kinetic Studies of the Reactions of Oxide, Hydroxide, Alkoxide, Phenyl, and Benzylic Anions with Methyl Chloride in the Gas Phase at 22.5'
7354 Kinetic Studies of the Reactions of Oxide, Hydroxide, Alkoxide, Phenyl, and Benzylic Anions with Methyl Chloride in the Gas Phase at 22.5' Diethard K. Bohme" and L. Brewster Young Contribution from the Centre for Research in Experimental Space Science, York Unicersity, Downsciew, Toronto, Canada, and Mobil Chemical Company, Edison, New Jersey 0881 7. Received June 15, 1970 Abstract: As part of a program directed toward an understanding of the intrinsic nature of anion-molecule re- actions involving organic constituents, rate constants have been measured, reaction channels have been identified, and intrinsic reaction probabilities (the ratios of the experimentally determined rate constants to the theoretical collision rate constants) have been calculated for the reactions of oxide, hydroxide, alkoxide, phenyl, and benzylic anions with methyl chloride. All measurements were made in the gas phase at 22.5" under thermal equilibrium conditions using the flowing afterglow technique. The highly charge-localized anions were found to react rapidly with methyl chloride with rate constants larger than 8 x 10-10 cm3molecule-1 sec-1 whereas the charge-delocalized anions reacted only slowly with rate constants less than 3 x 10-11 cm3molecule-1 sec-l. The formation of chloride ion was the dominant reaction channel observed in all cases. Calculated reaction probabilities were larger than 0.20 for the reactions involving highly charge-localized anions and less than 0.03 for the charge-delocalized anion re- actions. These results obtained in a rarified medium provide evidence for a correlation well known from solution studies, namely, a correlation between the reactivity of an anion and the nature of the distribution of its charge. -

Ultra Fine Powder from Alloy Resources Via Alkoxide.(Resources
High Temperature Materials and Processes, Vol. 8, No. 1, 1988, 39-46 Ultra Fine Powder from Alloy Resources via Alkoxide. (Resources Chain and Primary Innovation) by Nobuaki Sato and Michio Nanjo Research Institute of Mineral Dressing and Metallurgy, (SENKEN) Tohoku University, Sendai 980, Japan CONTENTS Page ABSTRACT 40 1. INTRODUCTION 40 2. FERRONIOBIUM PRODUCTION 40 3. CHLORINATION OF FERRONIOBIUM AND CHLORIDE SEPARATION 41 4. Nb-Ti SUPERCONDUCTOR SCRAP AS Nb SECONDARY RESOURCES 42 5. SYNTHESIS AND SEPARATION OF ALKOXIDES 43 6. PRODUCTION OF ULTRA-FINE POWDER VIA ALKOXIDE 45 7. PROPERTIES OF UFP Nb205 45 8. CONCLUDING REMARKS 46 ISSN 0334-1704 ©1988 by Freund Publishing House Ltd. 39 Vol. 8, No. 1, 1988 Ultra Fine Powder from Alloy Resources via Alkoxide ABSTRACT The urgent problem is to develop ore processing (first grade purification) to a high level (composition An attempt has been made to combine resource control, super purification) by developing super-refin- chains and primary innovation in high technology ing processes which facilitate process expansion, from fields. Alloy materials from urban mines are discussed alloys to new material applications in high-tech fields. as a new type of rare metal resource. Recovering nio- In this paper, we try to develop a new material, i.e. bium from ferroniobium and Nb-Ti superconducting ultrafine oxide powder (function development) from alloy via chloride methods has been studied. The ferroniobium or Nb-Ti scrap via chlorine metallurgy, production process of alkoxides from alloy resources alkoxide synthesis and purification (organic), and dis- has been investigated. Ultra fine NbiOs powder was cuss the resources and metallurgy for future research. -

Polymerization of Methyl and Ethyl Methacrylates Initiated with Alkali-Metal Alkoxide Derivatives of Poly(Ethylene Oxide)
Polymer Journal, Vol. 8, No. 2, pp 190-195 (1976) Polymerization of Methyl and Ethyl Methacrylates Initiated with Alkali-Metal Alkoxide Derivatives of Poly(ethylene oxide) Masao TOMOI, Yoshio SHIBA Y AMA, and Hiroshi KAKIUCHI Department of Applied Chemistry, Faculty of Engineering, Yokohama National University, Minami-ku, Yokohama 233, Japan. (Received September 2, 1975) ABSTRACT: The polymerization of methacrylates with alkali-metal alkoxide de rivatives of poly( ethylene oxide) (PEO) yielded copolymers which consisted of PEO and polymethacrylates containing block and graft units. The block copolymerization was initiated by addition of the alkoxide to the carbon-carbon double bond of methacrylates, and the graft reaction onto the block copolymer formed was thought to proceed by transesterification between the pendant ester groups of the copolymer and the unreacted alkoxide. The grafting of the alkoxide derivative of PEO having two terminal hydroxyl groups resulted in formation of cross-linked copolymers, while the polymerization with the alkoxide having one active end gave soluble copolymers. The relative amount of the grafted ester groups in the copolymers was estimated by NMR spectroscopy. KEY WORDS Anionic Polymerization I Block Copolymer I Graft Co- polymer I Poly(ethylene oxide) I Alkyl Methacrylate 1 Alkali-Metal Alkoxide I Crosslinking I Transesterification 1 In a previous paper/ it was shown that the (MMA and EMA) with macromolecular initiators polymerization of methyl methacrylate (MMA) (alkoxide derivatives of PEO). Such block co with alkali-metal salts of 2-substituted ethanols polymers, which consist of alkylene oxide and such as 2-methoxyethanol and 2-dimethylamino vinyl or diene monomers, have already been ethanol proceeded easily in the presence of synthesized by radical copolymerization, 4 but hexamethylphosphoramide (HMPA).