8.6 Acidity of Alcohols and Thiols 355
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anaerobic Degradation of Methanethiol in a Process for Liquefied Petroleum Gas (LPG) Biodesulfurization
Anaerobic degradation of methanethiol in a process for Liquefied Petroleum Gas (LPG) biodesulfurization Promotoren Prof. dr. ir. A.J.H. Janssen Hoogleraar in de Biologische Gas- en waterreiniging Prof. dr. ir. A.J.M. Stams Persoonlijk hoogleraar bij het laboratorium voor Microbiologie Copromotor Prof. dr. ir. P.N.L. Lens Hoogleraar in de Milieubiotechnologie UNESCO-IHE, Delft Samenstelling promotiecommissie Prof. dr. ir. R.H. Wijffels Wageningen Universiteit, Nederland Dr. ir. G. Muyzer TU Delft, Nederland Dr. H.J.M. op den Camp Radboud Universiteit, Nijmegen, Nederland Prof. dr. ir. H. van Langenhove Universiteit Gent, België Dit onderzoek is uitgevoerd binnen de onderzoeksschool SENSE (Socio-Economic and Natural Sciences of the Environment) Anaerobic degradation of methanethiol in a process for Liquefied Petroleum Gas (LPG) biodesulfurization R.C. van Leerdam Proefschrift ter verkrijging van de graad van doctor op gezag van de rector magnificus van Wageningen Universiteit Prof. dr. M.J. Kropff in het openbaar te verdedigen op maandag 19 november 2007 des namiddags te vier uur in de Aula Van Leerdam, R.C., 2007. Anaerobic degradation of methanethiol in a process for Liquefied Petroleum Gas (LPG) biodesulfurization. PhD-thesis Wageningen University, Wageningen, The Netherlands – with references – with summaries in English and Dutch ISBN: 978-90-8504-787-2 Abstract Due to increasingly stringent environmental legislation car fuels have to be desulfurized to levels below 10 ppm in order to minimize negative effects on the environment as sulfur-containing emissions contribute to acid deposition (‘acid rain’) and to reduce the amount of particulates formed during the burning of the fuel. Moreover, low sulfur specifications are also needed to lengthen the lifetime of car exhaust catalysts. -

Category Name C2-C4 Aliphatic Thiols Category Chemical Names
SIAM 30, 20-22 April 2010 US/ICCA Category Name C2-C4 Aliphatic Thiols Category 1-Ethanethiol (CAS No. 75-08-1) Chemical Names 1-Propanethiol (CAS No.107-03-9) and CAS Nos. 1-Butanethiol (CAS No.109-79-5) 2-Propanethiol, 2-Methyl (CAS No. 75-66-1) H2 H2 C HS C C CH HS CH3 3 H2 1-Ethanethiol 1-Propanethiol (Ethyl Mercaptan) (n-Propyl Mercaptan) H H CH Structural Formulae 2 2 3 C C HS C CH 3 H3C SH H2 1-Butanethiol CH3 (n-Butyl Mercaptan) 2-Propanethiol, 2-Methyl (t-Butyl Mercaptan) SUMMARY CONCLUSIONS OF THE SIAR Category Rationale The C2-C4 Aliphatic Thiols contain a sulfhydryl (SH) functional group with a straight or branched aliphatic carbon chain that characterizes the category. The four aliphatic thiols are soluble in water and have reasonably comparable melting points, initial boiling points and vapor pressures, as well as very low and objectionable odor thresholds. The water solubility and narrow range of octanol-water partition coefficients (log Kow) for the three linear C2-C4 Aliphatic Thiols indicate that they will have similar environmental fate and are not expected to bioaccumulate in aquatic organisms. Ecotoxicity is similar for the three linear C2-C4 Aliphatic Thiols with data for fish, invertebrate and algae toxicity indicating a similar order of acute toxicity across the chemicals tested (ecotoxicity is less for t-butyl-mercaptan). ECOSAR has been used to address and support the data gaps for the linear category members. Environmental fate and toxicity data are available for the branched t-butyl mercaptan. -

United States Patent Office Patented Nov
3,221,026 United States Patent Office Patented Nov. 30, 1965 2 3,221,026 prepared by reaction of a dicyanoketene acetal of the SALTS OF 1,1-DCYANO-2,2,2-TRIALKOXY formula ETHANES Owen W. Webster, Wilmington, Del, assignor to E. I. du Pont de Nemours and Company, Wilmington, Del., a corporation of Delaware No Drawing. Filed Feb. 13, 1962, Ser. No. 172,875 wherein R2 and R3 have the meanings defined above in the 2 Claims. (C. 260-340.9) general formula for the products of this invention, with This invention relates to salts of polycyano compounds, one molar equivalent of an alkali metal alkoxide of an and more particularly, to salts of polycyanopolyalkoxy alcohol having 1-8 carbon atoms at a temperature below ethanes and a process for their preparation. 10° C., and preferably at a temperature between 0 and The salts are derivatives of tetracyanoethylene which -80° C., in the presence of an inert reaction medium, is a very reactive compound that has received considerable e.g., an excess of the alcohol from which the alkoxide is study during the last few years. A large number of new 5 derived, or an ether such as diethyl ether, dioxane, tetra and valuable compounds have been prepared from it, and hydrofuran, ethylene glycol dimethyl ether and the like. now a new class of polycyano compounds is provided by As in the case of the reaction starting with tetracyano the present invention. ethylene, the reaction mixture in this case should also The novel compounds of this invention are salts of the be anhydrous to obtain the best results. -

Synthesis of Novel Single-Source Precursors for CVD of Mixed-Metal Tungsten Oxide
Synthesis of novel single-source precursors for CVD of mixed-metal tungsten oxide Hamid Choujaa A thesis submitted for the degree of Doctor of Philosophy University of Bath Department of Chemistry March 2008 COPYRIGHT Attention is drawn to the fact that copyright of this thesis rests with its author. This copy of the thesis has been supplied on condition that anyone who consults it is understood to recognize that its copyright rests with its author and that no quotation the thesis and no information derived from it may be published without the prior written consent of the author. This thesis may be made available for consultation within the University Library and may be photocopied or lent to other libraries for the purposes of consultation. TABLE OF CONTENTS Abstract ....................................................................................................................................... i Acknowledgements .................................................................................................................... iii Abbreviations and Acronyms .................................................................................................... iv 1. INTRODUCTION .................................................................................................................. 1 1.1 Generality about tungsten(VI) oxide ............................................................................. 1 1.1.1 The different lattice structures of tungsten oxide ........................................... 1 1.1.2 Electronic and -

Nitric Oxide Activation Facilitated by Cooperative Multimetallic Electron Transfer Within an Iron- Cite This: Chem
Chemical Science View Article Online EDGE ARTICLE View Journal | View Issue Nitric oxide activation facilitated by cooperative multimetallic electron transfer within an iron- Cite this: Chem. Sci.,2018,9,6379 – † All publication charges for this article functionalized polyoxovanadate alkoxide cluster have been paid for by the Royal Society a a a a b of Chemistry F. Li, R. L. Meyer, S. H. Carpenter, L. E. VanGelder, A. W. Nichols, C. W. Machan, b M. L. Neidiga and E. M. Matson *a A series of NO-bound, iron-functionalized polyoxovanadate–alkoxide (FePOV–alkoxide) clusters have been synthesized, providing insight into the role of multimetallic constructs in the coordination and activation of a substrate. Upon exposure of the heterometallic cluster to NO, the vanadium-oxide metalloligand is oxidized by a single electron, shuttling the reducing equivalent to the {FeNO} subunit to 7 V IV form a {FeNO} species. Four NO-bound clusters with electronic distributions ranging from [V3V2 ] Received 1st March 2018 {FeNO}7 to [VIV]{FeNO}7 have been synthesized, and characterized via 1H NMR, infrared, and electronic Accepted 30th June 2018 5 absorption spectroscopies. The ability of the FePOV–alkoxide cluster to store reducing equivalents in the DOI: 10.1039/c8sc00987b Creative Commons Attribution 3.0 Unported Licence. metalloligand for substrate coordination and activation highlights the ultility of the metal-oxide scaffold rsc.li/chemical-science as a redox reservoir. Introduction The activation of NO requires the simultaneous transfer of multiple electrons and protons to the substrate. In nature, The chemical reactivity of nitric oxide (NO) has captivated the eld similar chemical transformations of gaseous substrates (e.g. -

Direct Synthesis of Some Significant Metal Alkoxides
SD0000032 DIRECT SYNTHESIS OF SOME SIGNIFICANT METAL ALKOXI'DE BVYU EMI1JG A THESIS SUBMITTED FOR THE DEGREE OF Mi.Sc. IN CHEMISTRY SUPERVISOR: Dr. O.Y.OMER DEPARTMENT OF CHEMISTRY FACULTY OF EDUCATION UNIVERSITY OF KHARTOUM NOVEMBER, 1998 31/28 DISCLAIMER Portions of this document may be illegible in electronic image products. Images are produced from the best available original document. Please be aware that all of the Missing Pages in this document were originally blank pages Dedication To my three children: Regina, Maria and Samuel CONTENTS Page Dedication i Contents ii List of Tables v List of Figures vii Acknowledgement viii Abstract (Arabic) ix Abstract (English) x CHAPTER 1.0 CHAPTER ONE - INTRODUCTION 1 2.0 CHAPTER TWO - LITERATURE REVIEW 5 2.1 Introduction to Literature Review 5 2.2 Definition of metal alkoxides 5 2.3 Metal elements and (heir chemistry 8 2.3.1 Sodium metal 8 2.3.2 Magnesium metal 12 2.3.3 Aluminium metal 16 2.3.3.1 Hydrolysis of aluminium compounds 20 2.3.4 Tin metal 21 2.4 Preparative methods and uses ofalkoxides ofNa, Mg, Al & Sn. 25 2.4.1 Sodium alkoxide 25 2.4.2 Mamiesium alkoxide 26 2.4.3 Aluminium alkoxide 27 2.4.4. Tin alkoxide 30 2.5 General properties of metal alkoxides 31 2.5.1 1 lydrolysis in metal alkoxide 34 3.0 CHAPTER THREE - MATERIALS AND EXPERIMENTAL PROCEDURE 36 3.1 General procedures 36 3.1.1 Start ing material s 3 6 3.1. I.I Apparatus 36 3.1.1.2 Dry ethanol and isopropanol 36 3.1.1.3 Na, Mg, Al & Sn metals 36 3.1.2 Infrared spectra (Ir) 37 3.2 Reactions procedures 37 3.2.1 Reaction between sodium metal and absolute ethanol 37 3.2.2 Reaction of magnesium metal with absolute ethanol 37 3.2.3 Reaction of magnesium mclal with absolute ethanol using mercury (11) chloride catalyst. -

THIOL OXIDATION a Slippery Slope the Oxidation of Thiols — Molecules RSH Oxidation May Proceed Too Predominates
RESEARCH HIGHLIGHTS Nature Reviews Chemistry | Published online 25 Jan 2017; doi:10.1038/s41570-016-0013 THIOL OXIDATION A slippery slope The oxidation of thiols — molecules RSH oxidation may proceed too predominates. Here, the maximum of the form RSH — can afford quickly for intermediates like RSOH rate constants indicate the order − − − These are many products. From least to most to be spotted and may also afford of reactivity: RSO > RS >> RSO2 . common oxidized, these include disulfides intractable mixtures. Addressing When the reactions are carried out (RSSR), as well as sulfenic (RSOH), the first problem, Chauvin and Pratt in methanol-d , the obtained kinetic reactions, 1 sulfinic (RSO2H) and sulfonic slowed the reactions down by using isotope effect values (kH/kD) are all but have (RSO3H) acids. Such chemistry “very sterically bulky thiols, whose in the range 1.1–1.2, indicating that historically is pervasive in nature, in which corresponding sulfenic acids were no acidic proton is transferred in the been very disulfide bonds between cysteine known to be isolable but were yet rate-determining step. Rather, the residues stabilize protein structures, to be thoroughly explored in terms oxidations involve a specific base- difficult to and where thiols and thiolates often of reactivity”. The second problem catalysed mechanism wherein an study undergo oxidation by H2O2 or O2 in was tackled by modifying the model acid–base equilibrium precedes the order to protect important biological system, 9-mercaptotriptycene, by rate-determining nucleophilic attack − − − structures from damage. Among including a fluorine substituent to of RS , RSO or RSO2 on H2O2. the oxidation products, sulfenic serve as a spectroscopic handle. -

United States Patent Office Patented May 7, 1963
3,088,959 United States Patent Office Patented May 7, 1963 1. 2 or grouping of carbon atoms which is present in cyclo 3,088,959 pentadiene. This grouping is illustrated as PROCESS OF MAKENG CYCLOPENTADEENY NECKEL, NTROSYL COMPOUNDS Robert D. Feltham, Joseph F. Anzenberger, azad Jonatian T. Carrie, Pittsburgh, Pa., assignors to The Interaa tional Nickel Company, Inc., New York, N.Y., a corpo ration of Delaware No Drawing. FiRed Sept. 1, 1960, Ser. No. 53,374 The substituent groups on the cyclopentadiene moiety 6 Clains. (C. 260-439) 0. indicated as R, R2, R3, R and R5 are any one or more The present invention relates to the production of of hydrogen atoms, halogen atoms and/or organic groups nickel compounds and, more particularly, to the produc such as aliphatic groups, aromatic groups, alicyclic groups, tion of nickel nitrosyl compounds containing a group etc. The substituent groups can also bond at two posi having the cyclopentadienyl moiety. tions. Where this occurs, groups can substitute for adja Compounds such as cyclopentadienylnickel nitrosyl, 5 cent R groups, e.g., Ra and R3 and/or R4 and R5 to form methylcyclopentadienylnickel nitrosyl and other complex indene and other condensed ring structures. nitrosyl compounds containing a cyclopentadienyl-type As mentioned hereinbefore, when carrying out the proc group have been made. Such compounds have use as ess of the present invention, the reactants are reacted in gasoline additives. When such use is contemplated, it is the presence of a base. The base can advantageously be economically imperative that the compounds be produced 20 a nitrogen base or a phosphorus base or an alkoxide of a in good yield from the most readily available and inex metal having a strong hydroxide. -
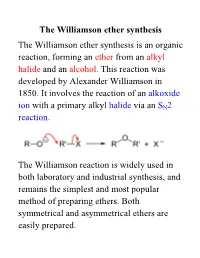
Williamson Ether Synthesis the Williamson Ether Synthesis Is an Organic Reaction, Forming an Ether from an Alkyl Halide and an Alcohol
The Williamson ether synthesis The Williamson ether synthesis is an organic reaction, forming an ether from an alkyl halide and an alcohol. This reaction was developed by Alexander Williamson in 1850. It involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction. The Williamson reaction is widely used in both laboratory and industrial synthesis, and remains the simplest and most popular method of preparing ethers. Both symmetrical and asymmetrical ethers are easily prepared. The reaction for this week: an example of a Williamson ether synthesis acetaminophen ethyl iodide phenacetin starting material reagent product Phenacetin may be synthesized as an example of the Williamson ether synthesis The first synthesis of phenacetin was reported in 1878 by Harmon Morse. Procedure 1. Weigh an Extra-Strength Tylenol tablet. Pulverize the tablet with mortar and pestle. Weigh out 0.22 g and place it in a dry 15-ml round-bottom flask along with 0.28 g of finely pulverized K2CO3 (mortar and pestle) and 3.0 mL of butanone. Carefully add 0.28 mL of ethyl iodide with a syringe. 2. Add a stir bar; attach a microscale water-cooled condenser to the flask. Heat the mixture under reflux directly on a hot plate at medium setting for 1 hour. In the meantime, obtain the IR of acetaminophen. 3. Turn off the heat. Allow the mixture to cool down. Add 4 mL of water to the flask and transfer its contents to a 16 x 125 mm test tube with a screw cap. Rinse round-bottom flask 4 times with 1 mL of tert-butyl methyl ether (BME) and add the rinsings to the test tube. -

Ethyl Mercaptan, Final AEGL Document
This PDF is available from The National Academies Press at http://www.nap.edu/catalog.php?record_id=18449 Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 15 ISBN Committee on Acute Exposure Guideline Levels; Committee on 978-0-309-29122-4 Toxicology; Board on Environmental Studies and Toxicology; Division on Earth and Life Studies; National Research Council 294 pages 6 x 9 PAPERBACK (2013) Visit the National Academies Press online and register for... Instant access to free PDF downloads of titles from the NATIONAL ACADEMY OF SCIENCES NATIONAL ACADEMY OF ENGINEERING INSTITUTE OF MEDICINE NATIONAL RESEARCH COUNCIL 10% off print titles Custom notification of new releases in your field of interest Special offers and discounts Distribution, posting, or copying of this PDF is strictly prohibited without written permission of the National Academies Press. Unless otherwise indicated, all materials in this PDF are copyrighted by the National Academy of Sciences. Request reprint permission for this book Copyright © National Academy of Sciences. All rights reserved. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 15 Committee on Acute Exposure Guideline Levels Committee on Toxicology Board on Environmental Studies and Toxicology Division on Earth and Life Studies Copyright © National Academy of Sciences. All rights reserved. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 15 THE NATIONAL ACADEMIES PRESS 500 FIFTH STREET, NW WASHINGTON, DC 20001 NOTICE: The project that is the subject of this report was approved by the Governing Board of the National Research Council, whose members are drawn from the councils of the National Academy of Sciences, the National Academy of Engineering, and the Insti- tute of Medicine. -

SAFETY DATA SHEET Santa Cruz Biotechnology, Inc
SAFETY DATA SHEET Santa Cruz Biotechnology, Inc. Revision date 23-Dec-2016 Version 1 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND OF THE COMPANY/UNDERTAKING _____________________________________________________________________________________________________________________ Product identifier Product Name Ethanethiol Product Code SC-239867 Recommended use of the chemical and restrictions on use For research use only. Not intended for diagnostic or therapeutic use. Details of the supplier of the safety data sheet Emergency telephone number Santa Cruz Biotechnology, Inc. Chemtrec 10410 Finnell Street 1.800.424.9300 (Within USA) Dallas, TX 75220 +1.703.527.3887 (Outside USA) 831.457.3800 800.457.3801 [email protected] 2. HAZARDS IDENTIFICATION _____________________________________________________________________________________________________________________ This chemical is considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification Acute toxicity - Oral Category 4 Acute toxicity - Dermal Category 4 Acute toxicity - Inhalation (Dusts/Mists) Category 4 Flammable liquids Category 1 Label elements Signal word Danger Hazard statements Harmful if swallowed Harmful in contact with skin Harmful if inhaled Extremely flammable liquid and vapor Symbols/Pictograms Precautionary Statements - Prevention Wash face, hands and any exposed skin thoroughly after handling Do not eat, drink or smoke when using this product Wear protective gloves/protective clothing/eye protection/face protection Avoid breathing -

Thiolated Polymers: Stability of Thiol Moieties Under Different Storage Conditions
Scientia Pharmaceutica (Sci. Pharm.) 70, 331-339 (2002) 0 Osterreichische Apotheker-Verlagsgesellschaftm.b.H., Wien, Printed in Austria Thiolated polymers: Stability of thiol moieties under different storage conditions A. ~ernko~-schnijrchi*,M.D. ~ornof'*,C.E. ~ast'and N. ~angoth' 'institute of Pharmaceutical Technology and Biopharmaceutics, Center of Pharmacy, University of Vienna, Althanstr. 14, 1090 Vienna, Austria 2~romaPharma GmbH, lndustriezeile 6, 2100 Leobendorf, Austria Abstract: The purpose of this study was to evaluate the stability of thiolated polymers - so-called thiomers. A polycarbophil-cysteine conjugate and a chitosan-thioglycolic acid conjugate were chosen as representative anionic and cationic thiomer. The thiol group bearing compounds L-cysteine and thioglycolic acid were introduced to polycarbophil and chitosan, respectively with a coupling reaction mediated by a carbodiimide. The resulting thiolated polymers were freeze-dried and the amount of thiol groups on the thiomer was determined spectrophotometrically. Each kind of polymer was directly used or compressed into 1 mg matrix-tablets. Polymers were stored for a period of six months at four different storage conditions, namely at -20°C (56% relative humidity; RH), 4°C (53% RH), at 20°C (70% RH), and at 22°C (25% RH). Samples were taken after 6 months to determine the formation of disulfide bonds and the remaining thiol groups on the polymer. When the polycarbophil-cysteine and chitosan-thioglycolic acid conjugate were stored as powder a decrease of free thiol groups was observed only after storage at 20°C and 70% RH. Both polymers were found to be stable under all storage conditions when compressed into matrix tablets.