United States Patent Office Patented May 7, 1963
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

United States Patent Office Patented Nov
3,221,026 United States Patent Office Patented Nov. 30, 1965 2 3,221,026 prepared by reaction of a dicyanoketene acetal of the SALTS OF 1,1-DCYANO-2,2,2-TRIALKOXY formula ETHANES Owen W. Webster, Wilmington, Del, assignor to E. I. du Pont de Nemours and Company, Wilmington, Del., a corporation of Delaware No Drawing. Filed Feb. 13, 1962, Ser. No. 172,875 wherein R2 and R3 have the meanings defined above in the 2 Claims. (C. 260-340.9) general formula for the products of this invention, with This invention relates to salts of polycyano compounds, one molar equivalent of an alkali metal alkoxide of an and more particularly, to salts of polycyanopolyalkoxy alcohol having 1-8 carbon atoms at a temperature below ethanes and a process for their preparation. 10° C., and preferably at a temperature between 0 and The salts are derivatives of tetracyanoethylene which -80° C., in the presence of an inert reaction medium, is a very reactive compound that has received considerable e.g., an excess of the alcohol from which the alkoxide is study during the last few years. A large number of new 5 derived, or an ether such as diethyl ether, dioxane, tetra and valuable compounds have been prepared from it, and hydrofuran, ethylene glycol dimethyl ether and the like. now a new class of polycyano compounds is provided by As in the case of the reaction starting with tetracyano the present invention. ethylene, the reaction mixture in this case should also The novel compounds of this invention are salts of the be anhydrous to obtain the best results. -

Synthesis of Novel Single-Source Precursors for CVD of Mixed-Metal Tungsten Oxide
Synthesis of novel single-source precursors for CVD of mixed-metal tungsten oxide Hamid Choujaa A thesis submitted for the degree of Doctor of Philosophy University of Bath Department of Chemistry March 2008 COPYRIGHT Attention is drawn to the fact that copyright of this thesis rests with its author. This copy of the thesis has been supplied on condition that anyone who consults it is understood to recognize that its copyright rests with its author and that no quotation the thesis and no information derived from it may be published without the prior written consent of the author. This thesis may be made available for consultation within the University Library and may be photocopied or lent to other libraries for the purposes of consultation. TABLE OF CONTENTS Abstract ....................................................................................................................................... i Acknowledgements .................................................................................................................... iii Abbreviations and Acronyms .................................................................................................... iv 1. INTRODUCTION .................................................................................................................. 1 1.1 Generality about tungsten(VI) oxide ............................................................................. 1 1.1.1 The different lattice structures of tungsten oxide ........................................... 1 1.1.2 Electronic and -

8.6 Acidity of Alcohols and Thiols 355
08_BRCLoudon_pgs5-1.qxd 12/8/08 11:05 AM Page 355 8.6 ACIDITY OF ALCOHOLS AND THIOLS 355 ural barrier to the passage of ions. However, the hydrocarbon surface of nonactin allows it to enter readily into, and pass through, membranes. Because nonactin binds and thus transports ions, the ion balance crucial to proper cell function is upset, and the cell dies. Ion Channels Ion channels, or “ion gates,” provide passageways for ions into and out of cells. (Recall that ions are not soluble in membrane phospholipids.) The flow of ions is essen- tial for the transmission of nerve impulses and for other biological processes. A typical chan- nel is a large protein molecule imbedded in a cell membrane. Through various mechanisms, ion channels can be opened or closed to regulate the concentration of ions in the interior of the cell. Ions do not diffuse passively through an open channel; rather, an open channel contains regions that bind a specific ion. Such an ion is bound specifically within the channel at one side of the membrane and is somehow expelled from the channel on the other side. Remark- ably, the structures of the ion-binding regions of these channels have much in common with the structures of ionophores such as nonactin. The first X-ray crystal structure of a potassium- ion channel was determined in 1998 by a team of scientists at Rockefeller University led by Prof. Roderick MacKinnon (b. 1956), who shared the 2003 Nobel Prize in Chemistry for this work. The interior of the channel contains binding sites for two potassium ions; these sites are oxygen-rich, much like the interior of nonactin. -

A Novel Series of Titanocene Dichloride Derivatives: Synthesis, Characterization and Assessment of Their
A novel series of titanocene dichloride derivatives: synthesis, characterization and assessment of their cytotoxic properties by Gregory David Potter A thesis submitted to the Department of Chemistry in conformity with the requirements for the degree of Doctor of Philosophy Queen’s University Kingston, Ontario, Canada May, 2008 Copyright © Gregory David Potter, 2008 Abstract Although cis-PtCl2(NH3)2 (cisplatin) has been widely used as a chemotherapeutic agent, its use can be accompanied by toxic side effects and the development of drug resistance. Consequently, much research has been focused on the discovery of novel transition metal compounds which elicit elevated cytotoxicities coupled with reduced toxic side effects and non-cross resistance. Recently, research in this lab has focused on preparing derivatives of titanocene dichloride (TDC), a highly active chemotherapeutic agent, with pendant alkylammonium groups on one or both rings. Earlier results have demonstrated that derivatives containing either cyclic or chiral alkylammonium groups had increased cytotoxic activities. This research therefore investigated a new series of TDC complexes focusing specifically on derivatives bearing cyclic and chiral alkylammonium groups. A library of ten cyclic derivatives and six chiral derivatives were synthesized and fully characterized. These derivatives have undergone in vitro testing as anti-tumour agents using human lung, ovarian, and cervical carcinoma cell lines (A549, H209, H69, H69/CP, A2780, A2780/CP and HeLa). These standard cell lines represent solid tumour types for which new drugs are urgently needed. The potencies of all of the Ti (IV) derivatives varied greatly (range from 10.8 μM - >1000 μM), although some trends were observed. In general, the dicationic analogues exhibited greater potency than the corresponding monocationic derivatives. -

Catalysis Science & Technology
Catalysis Science & Technology Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. We will replace this Accepted Manuscript with the edited and formatted Advance Article as soon as it is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/catalysis Page 1 of 6 CatalysisPlease doScience not adjust & Technology margins Journal Name ARTICLE Hydrophenylation of internal alkynes with boronic acids catalysed by a Ni-Zn hydroxy double salt-intercalated Received 00th January 20xx, Accepted 00th January 20xx Manuscript DOI: 10.1039/x0xx00000x anionic rhodium(III) complex www.rsc.org/ Takayoshi Hara, 1 Nozomi Fujita, 1 Nobuyuki Ichikuni, 1 Karen Wilson, 2 Adam F. Lee, 2 and Shogo Shimazu* 1 3- [Rh(OH) 6] intercalated Ni–Zn mixed basic salts (Rh/NiZn) are efficient catalysts for the hydrophenylation of internal alkynes with arylboronic acids under mild conditions. -

Nitric Oxide Activation Facilitated by Cooperative Multimetallic Electron Transfer Within an Iron- Cite This: Chem
Chemical Science View Article Online EDGE ARTICLE View Journal | View Issue Nitric oxide activation facilitated by cooperative multimetallic electron transfer within an iron- Cite this: Chem. Sci.,2018,9,6379 – † All publication charges for this article functionalized polyoxovanadate alkoxide cluster have been paid for by the Royal Society a a a a b of Chemistry F. Li, R. L. Meyer, S. H. Carpenter, L. E. VanGelder, A. W. Nichols, C. W. Machan, b M. L. Neidiga and E. M. Matson *a A series of NO-bound, iron-functionalized polyoxovanadate–alkoxide (FePOV–alkoxide) clusters have been synthesized, providing insight into the role of multimetallic constructs in the coordination and activation of a substrate. Upon exposure of the heterometallic cluster to NO, the vanadium-oxide metalloligand is oxidized by a single electron, shuttling the reducing equivalent to the {FeNO} subunit to 7 V IV form a {FeNO} species. Four NO-bound clusters with electronic distributions ranging from [V3V2 ] Received 1st March 2018 {FeNO}7 to [VIV]{FeNO}7 have been synthesized, and characterized via 1H NMR, infrared, and electronic Accepted 30th June 2018 5 absorption spectroscopies. The ability of the FePOV–alkoxide cluster to store reducing equivalents in the DOI: 10.1039/c8sc00987b Creative Commons Attribution 3.0 Unported Licence. metalloligand for substrate coordination and activation highlights the ultility of the metal-oxide scaffold rsc.li/chemical-science as a redox reservoir. Introduction The activation of NO requires the simultaneous transfer of multiple electrons and protons to the substrate. In nature, The chemical reactivity of nitric oxide (NO) has captivated the eld similar chemical transformations of gaseous substrates (e.g. -

Synthesis and Reactions of Ferrocene
R Carbon Synthesis and Reactions of Ferrocene R Carbon Carbon Contents Objectives 1 Introduction 1 Preparation of ferrocene 2 Acetylation of ferrocene 7 Preparation of [Fe(η-C5H5)(η-C6H6)]PF6 10 Reaction of [Fe(η-C5H5)(η-C6H6)] PF6 with nucleophiles 12 Manuscript prepared by Dr. Almas I. Zayya, Dr. A. Jonathan Singh and Prof. John L. Spencer. School of Chemical and Physical Sciences, Victoria University of Wellington, New Zealand. R Carbon Objectives Introduction The principal aims of these experiments are to The archetypal organometallic compound provide experience in the synthesis, isolation, ferrocene, [Fe(η-C5H5)2], is of historical importance purification and characterisation of organometallic since its discovery and structural characterisation compounds. Purification techniques include in the early 1950s sparked extensive research into distillation, sublimation, chromatography and the chemistry of metal sandwich compounds.1 crystallisation. The main characterisation Two of the chemists who first proposed the correct technique used in these experiments is 1H NMR structure of ferrocene (Figure 1), Geoffrey Wilkinson spectroscopy using the benchtop Spinsolve and Ernst Otto Fischer, were awarded the Nobel spectrometer. Furthermore, students will Prize in Chemistry in 1973 for their pioneering work also develop their synthetic skills using inert on the chemistry of sandwich complexes. atmosphere techniques. Ferrocene is an example of a π-complex in which interactions between the d-orbitals of the Fe2+ metal centre with the π-orbitals of the two planar - cyclopentadienyl ligands (C5H5 ) form the metal-ligand bonds. Thus, all the carbon atoms in the cyclopentadienyl rings are bonded equally to the central Fe2+ ion. Ferrocene exhibits aromatic properties and is thermally very stable. -

Direct Synthesis of Some Significant Metal Alkoxides
SD0000032 DIRECT SYNTHESIS OF SOME SIGNIFICANT METAL ALKOXI'DE BVYU EMI1JG A THESIS SUBMITTED FOR THE DEGREE OF Mi.Sc. IN CHEMISTRY SUPERVISOR: Dr. O.Y.OMER DEPARTMENT OF CHEMISTRY FACULTY OF EDUCATION UNIVERSITY OF KHARTOUM NOVEMBER, 1998 31/28 DISCLAIMER Portions of this document may be illegible in electronic image products. Images are produced from the best available original document. Please be aware that all of the Missing Pages in this document were originally blank pages Dedication To my three children: Regina, Maria and Samuel CONTENTS Page Dedication i Contents ii List of Tables v List of Figures vii Acknowledgement viii Abstract (Arabic) ix Abstract (English) x CHAPTER 1.0 CHAPTER ONE - INTRODUCTION 1 2.0 CHAPTER TWO - LITERATURE REVIEW 5 2.1 Introduction to Literature Review 5 2.2 Definition of metal alkoxides 5 2.3 Metal elements and (heir chemistry 8 2.3.1 Sodium metal 8 2.3.2 Magnesium metal 12 2.3.3 Aluminium metal 16 2.3.3.1 Hydrolysis of aluminium compounds 20 2.3.4 Tin metal 21 2.4 Preparative methods and uses ofalkoxides ofNa, Mg, Al & Sn. 25 2.4.1 Sodium alkoxide 25 2.4.2 Mamiesium alkoxide 26 2.4.3 Aluminium alkoxide 27 2.4.4. Tin alkoxide 30 2.5 General properties of metal alkoxides 31 2.5.1 1 lydrolysis in metal alkoxide 34 3.0 CHAPTER THREE - MATERIALS AND EXPERIMENTAL PROCEDURE 36 3.1 General procedures 36 3.1.1 Start ing material s 3 6 3.1. I.I Apparatus 36 3.1.1.2 Dry ethanol and isopropanol 36 3.1.1.3 Na, Mg, Al & Sn metals 36 3.1.2 Infrared spectra (Ir) 37 3.2 Reactions procedures 37 3.2.1 Reaction between sodium metal and absolute ethanol 37 3.2.2 Reaction of magnesium metal with absolute ethanol 37 3.2.3 Reaction of magnesium mclal with absolute ethanol using mercury (11) chloride catalyst. -

Nickel in Drinking-Water (2005)
WHO/SDE/WSH/05.08/55 English only Nickel in Drinking-water Background document for development of WHO Guidelines for Drinking-water Quality © World Health Organization 2005 The illustration on the cover page is extracted from Rescue Mission: Planet Earth,© Peace Child International 1994; used by permission. This document may be freely reviewed, abstracted, reproduced and translated in part or in whole but not for sale or for use in conjunction with commercial purposes. Inquiries should be addressed to: [email protected]. The designations employed and the presentation of the material in this document do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. The World Health Organization does not warrant that the information contained in this publication is complete and correct and shall not be liable for any damages incurred as a result of its use. Preface One of the primary goals of WHO and its member states is that “all people, whatever their stage of development and their social and economic conditions, have the right to have access to an adequate supply of safe drinking water.” A major WHO function to achieve such goals is the responsibility “to propose .. -
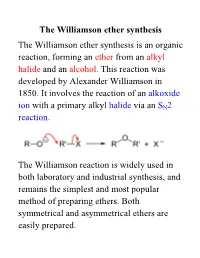
Williamson Ether Synthesis the Williamson Ether Synthesis Is an Organic Reaction, Forming an Ether from an Alkyl Halide and an Alcohol
The Williamson ether synthesis The Williamson ether synthesis is an organic reaction, forming an ether from an alkyl halide and an alcohol. This reaction was developed by Alexander Williamson in 1850. It involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction. The Williamson reaction is widely used in both laboratory and industrial synthesis, and remains the simplest and most popular method of preparing ethers. Both symmetrical and asymmetrical ethers are easily prepared. The reaction for this week: an example of a Williamson ether synthesis acetaminophen ethyl iodide phenacetin starting material reagent product Phenacetin may be synthesized as an example of the Williamson ether synthesis The first synthesis of phenacetin was reported in 1878 by Harmon Morse. Procedure 1. Weigh an Extra-Strength Tylenol tablet. Pulverize the tablet with mortar and pestle. Weigh out 0.22 g and place it in a dry 15-ml round-bottom flask along with 0.28 g of finely pulverized K2CO3 (mortar and pestle) and 3.0 mL of butanone. Carefully add 0.28 mL of ethyl iodide with a syringe. 2. Add a stir bar; attach a microscale water-cooled condenser to the flask. Heat the mixture under reflux directly on a hot plate at medium setting for 1 hour. In the meantime, obtain the IR of acetaminophen. 3. Turn off the heat. Allow the mixture to cool down. Add 4 mL of water to the flask and transfer its contents to a 16 x 125 mm test tube with a screw cap. Rinse round-bottom flask 4 times with 1 mL of tert-butyl methyl ether (BME) and add the rinsings to the test tube. -

Inorganic Arsenic Compounds Other Than Arsine Health and Safety Guide
OS INTERNATiONAL I'ROGRAMME ON CHEMICAL SAFETY Health and Safety Guide No. 70 INORGANIC ARSENIC COMPOUNDS OTHER THAN ARSINE HEALTH AND SAFETY GUIDE i - I 04 R. Q) UNEP UNITED NATIONS INTERNATIONAL ENVIRONMENT I'R( )GRAMME LABOUR ORGANISATION k\s' I V WORLD HEALTH ORGANIZATION WORLD HEALTH ORGANIZATION, GENEVA 1992 IPcs Other H EA LTH AND SAFETY GUIDES available: Aerytonitrile 41. Clii rdeon 2. Kekvau 42. Vatiadiuni 3 . I Bula not 43 Di meLhyI ftirmatnide 4 2-Buta101 44 1-Dryliniot 5. 2.4- Diehlorpheiioxv- 45 . Ac rylzi mule acetic Acid (2.4-D) 46. Barium 6. NIcihylene Chhride 47. Airaziiie 7 . ie,i-Buia nol 48. Benlm'.ie 8. Ep Ichioroli) Olin 49. Cap a 64 P. ls.ihutaiiol 50. Captaii I o. feiddin oeth N lene Si. Parai.tuat II. Tetradi ion 51 Diquat 12. Te nacelle 53. Alpha- and Betal-lexachloro- 13 Clils,i (lane cyclohexanes 14 1 kpia Idor 54. Liiidaiic IS. Propylene oxide 55. 1 .2-Diciilroetiiane Ethylene Oxide 5t. Hydrazine Eiulosiillaii 57. F-orivaldehydc IS. Die h lorvos 55. MLhyI Isobu I V I kcloiic IV. Pculaehloro1heiiol 59. fl-Flexaric 20. Diiiiethoaie 61), Endrin 2 1 . A iii in and Dick) 0in 6 I . I sh IIZiLI1 22. Cyperniellirin 62. Nicki. Nickel Caution I. and some 23. Quiiiloieiic Nickel Compounds 24. Alkthrins 03. Hexachlorocyclopeuladiene 25. Rsiiiethii ins 64. Aidicaib 26. Pyr rot ii,id inc Alkaloids 65. Fe nitrolhioit 27. Magnetic Fields hib. Triclilorlon 28. Phosphine 67. Acroleiii 29. Diiiiethyl Sull'ite 68. Polychlurinated hiphenyls (PCBs) and 30. Dc lianteth nil polyc h In ruiated letlilienyls (fs) 31. -

Nickel and Nickel Compounds Were Considered by Previous !AC Working Groups, in 1972, 1975, 1979, 1982 and 1987 (IARC, 1973, 1976, 1979, 1982, 1987)
NICKEL AND NieKEL eOMPOUNDS Nickel and nickel compounds were considered by previous !AC Working Groups, in 1972, 1975, 1979, 1982 and 1987 (IARC, 1973, 1976, 1979, 1982, 1987). Since that time, new data have become available, and these are inc1uded in the pres- ent monograph and have been taken into consideration in the evaluation. 1. ehemical and Physical Data The list of nickel alloys and compounds given in Table 1 is not exhaustive, nor does it necessarily reflect the commercial importance of the various nickel-con tain- ing substances, but it is indicative of the range of nickel alloys and compounds avail- able, including some compounds that are important commercially and those that have been tested in biological systems. A number of intermediary compounds occur in refineries which cannot be characterized and are not listed. 1.1 Synonyms, trade names and molecular formulae of nickel and selected nickel-containing compounds Table 1. Synonyms (Chemical Abstracts Service names are given in bold), trade names and atomic or molecular formulae or compositions of nickel, nickel alloys and selected nickel compounds Chemical Chem. Abstr. SYDoDyms and trade Dames Formula Dame Seiv. Reg. Oxda- Numbera tion stateb Metallc nickel and nickel alloys Nickel 7440-02-0 c.I. 77775; NI; Ni 233; Ni 270; Nickel 270; Ni o (8049-31-8; Nickel element; NP 2 17375-04-1; 39303-46-3; 53527-81-4; 112084-17-0) -- 257 - NICKEL AND NICKEL COMPOUNDS 259 Table i (contd) Chemical Chem. Abstr. Synonym and trade names Formula name Seiv. Reg. Ox- Number4 dation stateb