Carboxylic Acid Derivatives Carboxylic Acid Derivatives Are Described As Compounds That Can Be Converted to Carboxylic Acids Via Simple Acidic Or Basic Hydrolysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

De Novo Biosynthesis of Terminal Alkyne-Labeled Natural Products
De novo biosynthesis of terminal alkyne-labeled natural products Xuejun Zhu1,2, Joyce Liu2,3, Wenjun Zhang1,2,4* 1Department of Chemical and Biomolecular Engineering, 2Energy Biosciences Institute, 3Department of Bioengineering, University of California, Berkeley, CA 94720, USA. 4Physical Biosciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA. *e-mail:[email protected] 1 Abstract: The terminal alkyne is a functionality widely used in organic synthesis, pharmaceutical science, material science, and bioorthogonal chemistry. This functionality is also found in acetylenic natural products, but the underlying biosynthetic pathways for its formation are not well understood. Here we report the characterization of the first carrier protein- dependent terminal alkyne biosynthetic machinery in microbes. We further demonstrate that this enzymatic machinery can be exploited for the in situ generation and incorporation of terminal alkynes into two natural product scaffolds in E. coli. These results highlight the prospect for tagging major classes of natural products, including polyketides and polyketide/non-ribosomal peptide hybrids, using biosynthetic pathway engineering. 2 Natural products are important small molecules widely used as drugs, pesticides, herbicides, and biological probes. Tagging natural products with a unique chemical handle enables the visualization, enrichment, quantification, and mode of action study of natural products through bioorthogonal chemistry1-4. One prevalent bioorthogonal reaction is -

Phospholipid:Diacylglycerol Acyltransferase: an Enzyme That Catalyzes the Acyl-Coa-Independent Formation of Triacylglycerol in Yeast and Plants
Phospholipid:diacylglycerol acyltransferase: An enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants Anders Dahlqvist*†‡, Ulf Ståhl†§, Marit Lenman*, Antoni Banas*, Michael Lee*, Line Sandager¶, Hans Ronne§, and Sten Stymne¶ *Scandinavian Biotechnology Research (ScanBi) AB, Herman Ehles Va¨g 2 S-26831 Svaloˆv, Sweden; ¶Department of Plant Breeding Research, Swedish University of Agricultural Sciences, Herman Ehles va¨g 2–4, S-268 31 Svalo¨v, Sweden; and §Department of Plant Biology, Uppsala Genetic Center, Swedish University of Agricultural Sciences, Box 7080, S-750 07 Uppsala, Sweden Edited by Christopher R. Somerville, Carnegie Institution of Washington, Stanford, CA, and approved March 31, 2000 (received for review February 15, 2000) Triacylglycerol (TAG) is known to be synthesized in a reaction that acid) and epoxidated fatty acid (vernolic acid) in TAG in castor uses acyl-CoA as acyl donor and diacylglycerol (DAG) as acceptor, bean (Ricinus communis) and the hawk’s-beard Crepis palaestina, and which is catalyzed by the enzyme acyl-CoA:diacylglycerol respectively. Furthermore, a similar enzyme is shown to be acyltransferase. We have found that some plants and yeast also present in the yeast Saccharomyces cerevisiae, and the gene have an acyl-CoA-independent mechanism for TAG synthesis, encoding this enzyme, YNR008w, is identified. which uses phospholipids as acyl donors and DAG as acceptor. This reaction is catalyzed by an enzyme that we call phospholipid:dia- Materials and Methods cylglycerol acyltransferase, or PDAT. PDAT was characterized in Yeast Strains and Plasmids. The wild-type yeast strains used were microsomal preparations from three different oil seeds: sunflower, either FY1679 (MAT␣ his3-⌬200 leu2-⌬1 trp1-⌬6 ura3-52) (9) or castor bean, and Crepis palaestina. -

Chapter 21 the Chemistry of Carboxylic Acid Derivatives
Instructor Supplemental Solutions to Problems © 2010 Roberts and Company Publishers Chapter 21 The Chemistry of Carboxylic Acid Derivatives Solutions to In-Text Problems 21.1 (b) (d) (e) (h) 21.2 (a) butanenitrile (common: butyronitrile) (c) isopentyl 3-methylbutanoate (common: isoamyl isovalerate) The isoamyl group is the same as an isopentyl or 3-methylbutyl group: (d) N,N-dimethylbenzamide 21.3 The E and Z conformations of N-acetylproline: 21.5 As shown by the data above the problem, a carboxylic acid has a higher boiling point than an ester because it can both donate and accept hydrogen bonds within its liquid state; hydrogen bonding does not occur in the ester. Consequently, pentanoic acid (valeric acid) has a higher boiling point than methyl butanoate. Here are the actual data: INSTRUCTOR SUPPLEMENTAL SOLUTIONS TO PROBLEMS • CHAPTER 21 2 21.7 (a) The carbonyl absorption of the ester occurs at higher frequency, and only the carboxylic acid has the characteristic strong, broad O—H stretching absorption in 2400–3600 cm–1 region. (d) In N-methylpropanamide, the N-methyl group is a doublet at about d 3. N-Ethylacetamide has no doublet resonances. In N-methylpropanamide, the a-protons are a quartet near d 2.5. In N-ethylacetamide, the a- protons are a singlet at d 2. The NMR spectrum of N-methylpropanamide has no singlets. 21.9 (a) The first ester is more basic because its conjugate acid is stabilized not only by resonance interaction with the ester oxygen, but also by resonance interaction with the double bond; that is, the conjugate acid of the first ester has one more important resonance structure than the conjugate acid of the second. -

Ebook for A2.2 Chemistry
eBook for A2.2 Chemistry Chemistry in Medicine 5.11. [Pages 94 – 107 of A2.2 eBook] • You will be expected to be able to explain the use of indigestion remedies to cure excess hydrochloric acid in the stomach stating the types of compounds used and writing equations for their reactions. • You will be expected to be able to use a back titration to determine the percentage of an active ingredient in an indigestion remedy and perform various calculations on the titration. • You will be expected to be able to explain how to deal with variations in the pH values of skin, explain the role of fatty acids in skin pH and explain the use of corrosive chemicals in removing warts. • You will be expected to be able to recall and explain the use of silver nitrate in the treatment of eye diseases. • You will be expected to be able to explain the action of anticancer drugs, for example cisplatin in preventing DNA replication in cancer cells and how varying the structure of cisplatin affects the efficiency of anticancer activity • You will be expected to be able to carry out titrations to determine the concentration of aspirin in solution and carry out associated calculations. • You will be expected to be able to recall the synthesis of aspirin from salicylic acid and ethanoic anhydride and compare it with the use of ethanoic acid and ethanoyl chloride and explain why the sodium salt of aspirin is often used rather than aspirin. • You will be expected to be able to explain the use of GLC linked to MS to identify drugs and to determine their purity. -
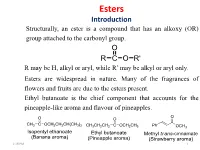
Esters Introduction Structurally, an Ester Is a Compound That Has an Alkoxy (OR) Group Attached to the Carbonyl Group
Esters Introduction Structurally, an ester is a compound that has an alkoxy (OR) group attached to the carbonyl group. O R C O R' R may be H, alkyl or aryl, while R’ may be alkyl or aryl only. Esters are widespread in nature. Many of the fragrances of flowers and fruits are due to the esters present. Ethyl butanoate is the chief component that accounts for the pineapple-like aroma and flavour of pineapples. 1:18 PM 1 Nomenclature of Esters Names of esters consist of two words that reflect the composite structure of the ester. The first word is derived from the alkyl group of the alcohol component, and the second word from the carboxylate group of the carboxylic acid component of the ester. The name of the carboxylate portion is derived by substituting the -ic acid suffix of the parent carboxylic acid with the –ate suffix. The alkyl group is cited first followed by the carboxylate group separated by a space. An ester is thus named as an alkyl 1:18 PM alkanoate. 2 IUPAC Nomenclature of Esters Examples 1:18 PM 3 Synthesis of Esters Preparative Strategies Highlighted below are some of the most common strategies by which esters are prepared. The esters are commonly prepared from the reaction of carboxylic acids, acid chlorides and acid anhydrides with alcohols. 1:18 PM 4 Synthesis of Esters Acid-Catalysed Esterification of a Carboxylic Acid and an Alcohol The acid-catalysed reaction of carboxylic acids and alcohols provides esters. Typically, a catalytic amount of a strong inorganic (mineral) acid such as H2SO4, HCl and H3PO4 is used. -

Derivatives of Carboxylic Acid
Derivatives of Carboxylic Acid acid chloride carboxylate nitrile amide acid anhydride ester Nomenclature of Acid Halides IUPAC: alkanoic acid → alkanoyl halide Common: alkanic acid → alkanyl halide I: 3-aminopropanoyl chloride I: 4-nitropentanoyl chloride c: b-aminopropionyl chloride c: g-nitrovaleryl chloride I: hexanedioyl chloride c: adipoyl chloride Rings: (IUPAC only): ringcarbonyl halide I: benzenecarbonyl bromide I: 3-cylcopentenecarbonyl chloride c: benzoyl bromide Nomenclature of Acid Anhydrides Acid anhydrides are prepared by dehydrating carboxylic acids acetic anhydride ethanoic acid ethanoic anhydride I: benzenecarboxylic anhydride I: butanedioic acid I: butanedioic anhydride c: benzoic andhydride c: succinic acid c: succinic anhydride Some unsymmetrical anhydrides I: ethanoic methanoic I: benzoic methanoic anhydride anhydride I: cis-butenedioic c: benzoic formic anhydride anhydride c: acetic formic anhydride Nomenclature of Esters Esters occur when carboxylic acids react with alcohols I: phenyl methanoate I: t-butyl benzenecarboxylate I: methyl ethanoate c: phenyl formate c: methyl acetate c: t-butyl benzoate I: isobutyl I: cyclobutyl 2- I: dimethyl ethanedioate cyclobutanecarboxylate methylpropanoate c: cyclobutyl a- c: dimethyl oxalate c: none methylpropionate Cyclic Esters Reaction of -OH and -COOH on same molecule produces a cyclic ester, lactone. To name, add word lactone to the IUPAC acid name or replace the -ic acid of common name with -olactone. 4-hydroxy-2-methylpentanoic acid lactone -methyl- -valerolactone Amides Product of the reaction of a carboxylic acid and ammonia or an amine. Not basic because the lone pair on nitrogen is delocalized by resonance. Classes of Amides 1 amide has one C-N bond (two N-H). 2 amide or N-substituted amide has two C-N bonds (one N-H). -

11 Carboxylic Acids and Derivatives
CARBOXYLIC ACIDS AND NITRILES Chapters 20, 21 Organic Chemistry, 8th Edition John McMurry 1 CARBOXYLIC ACID DERIVATIVES 2 CARBOXYLIC ACID DERIVATIVES R C N nitrile R = CH3 acetonitrile 3 STRUCTURE AND BONDING 4 NOMENCLATURE—THE IUPAC SYSTEM heptanoic acid benzoic acid cyclopentane carboxylic acid 4,5-dimethyl 3-pentenoic 3-bromobenzoic p-toluic 1-methyl- hexanoic acid acid acid acid cyclopropanecarboxylic acid 5 NOMENCLATURE-COMMON NAMES προτοσ πιον 6 NOMENCLATURE-POLYIACIDS Oxalis acetosella Malon Succinum HOOC COOH COOH propane-1,2,3-tricarboxylic acid 7 PHYSICAL PROPERTIES • Carboxylic acids exhibit dipole-dipole interactions because they have polar C—O and O—H bonds. • They also exhibit intermolecular hydrogen bonding. • In the gas phase and in apolar solvents, carboxylic acids often exist as dimers held together by two intermolecular hydrogen bonds. 8 PHYSICAL PROPERTIES 9 ACIDITY OF CARBOXYLIC ACIDS The acetate anion has two C—O bonds of equal length (1.27 Å) and intermediate between the length of a C—O single bond (1.36 Å) and C=O (1.21 Å). 10 CARBOXYLIC ACIDS—STRONG ORGANIC BRØNSTED-LOWRY ACIDS 11 THE INDUCTIVE EFFECT IN ALIPHATIC CARBOXYLIC ACIDS 12 THE INDUCTIVE EFFECT IN ALIPHATIC CARBOXYLIC ACIDS 13 SUBSTITUTED BENZOIC ACIDS 14 SUBSTITUTED BENZOIC ACIDS 15 PREPARATION OF CARBOXYLIC ACIDS [1] Oxidation of 1° alcohols [2] Oxidation of alkyl benzenes 16 PREPARATION OF CARBOXYLIC ACIDS [3] From alkyl halides CN CN- i. NaOH + ii.H3O Br COOH i. CO2 Mg + ii. H3O MgBr R MgBr R OMgBr H O+ R OH 3 + Mg++ + Br- O C O O O 17 PREPARATION OF CARBOXYLIC ACIDS [5] From alkyl halides. -

Fermentation and Ester Taints
Fermentation and Ester Taints Anita Oberholster Introduction: Aroma Compounds • Grape‐derived –provide varietal distinction • Yeast and fermentation‐derived – Esters – Higher alcohols – Carbonyls – Volatile acids – Volatile phenols – Sulfur compounds What is and Esters? • Volatile molecule • Characteristic fruity and floral aromas • Esters are formed when an alcohol and acid react with each other • Few esters formed in grapes • Esters in wine ‐ two origins: – Enzymatic esterification during fermentation – Chemical esterification during long‐term storage Ester Formation • Esters can by formed enzymatically by both the plant and microbes • Microbes – Yeast (Non‐Saccharomyces and Saccharomyces yeast) – Lactic acid bacteria – Acetic acid bacteria • But mainly produced by yeast (through lipid and acetyl‐CoA metabolism) Ester Formation Alcohol function Keto acid‐Coenzyme A Ester Ester Classes • Two main groups – Ethyl esters – Acetate esters • Ethyl esters = EtOH + acid • Acetate esters = acetate (derivative of acetic acid) + EtOH or complex alcohol from amino acid metabolism Ester Classes • Acetate esters – Ethyl acetate (solvent‐like aroma) – Isoamyl acetate (banana aroma) – Isobutyl acetate (fruit aroma) – Phenyl ethyl acetate (roses, honey) • Ethyl esters – Ethyl hexanoate (aniseed, apple‐like) – Ethyl octanoate (sour apple aroma) Acetate Ester Formation • 2 Main factors influence acetate ester formation – Concentration of two substrates acetyl‐CoA and fusel alcohol – Activity of enzyme responsible for formation and break down reactions • Enzyme activity influenced by fermentation variables – Yeast – Composition of fermentation medium – Fermentation conditions Acetate/Ethyl Ester Formation – Fermentation composition and conditions • Total sugar content and optimal N2 amount pos. influence • Amount of unsaturated fatty acids and O2 neg. influence • Ethyl ester formation – 1 Main factor • Conc. of precursors – Enzyme activity smaller role • Higher fermentation temp formation • C and N increase small effect Saerens et al. -

Amide, and Paratoluenesulfonamide on the Amide of Silver,On the Imides
68 CHEMISTRY: E. C. FRANKLIN METALLIC SALTS OF AMMONO ACIDS By Edward C. Franklin DEPARTMENT OF CHEMISTRY, STANFORD UNIVERSITY Presented to the Academy, January 9. 1915 The Action of Liquid-Ammonia Solutions of Ammono Acids on Metallic Amides, Imides, and Nitrides. The acid amides and imides, and the metallic derivatives of the acid amides and imides are the acds, bases, and salts respectively of an ammonia system of acids, bases, and salts.1 Guided by the relationships implied in the above statement Franklin and Stafford were able to prepare potassium derivatives of a considerable number of acid amides by the action of potassium amide on certain acid amides in solution in liquid ammonia. That is to say, an ammono base, potassium amide, was found to react with ammono acids in liquid ammonia to form ammono salts just as the aquo base, potassium hydrox- ide, acts upon aquo acids in water solution to form aquo salts. Choos- ing, for example, benzamide and benzoic acid as representative acids of the two systems, the analogous reactions taking place respectively in liquid ammonia and water are represented by the equations: CH6CONH2+KNH2 = C6H5CONHK + NHs. CseHCONH2 + 2KNH2 = CIHsCONK2 + 2NH3. CH6tCOOH + KOH = CIH6COOK + H2O. The ammono acid, since it is dibasic, reacts with either one or two molecules of potassium amide to form an acid and a neutral salt. Having thus demonstrated the possibility of preparing ammono salts of potassium by the interaction of potassium amide and acid amides in liquid ammonia solution, it was further found that ammono salts of the heavy metals may be prepared by the action of liquid ammonia solutions of ammono acids on insoluble metallic amides, imides, and nitrides-that is, by reactions which are analogous to the formation of aquo salts in water by the action of potassium hydroxide on insoluble metallic hydroxides and oxides. -

Amide Activation: an Emerging Tool for Chemoselective Synthesis
Featuring work from the research group of Professor As featured in: Nuno Maulide, University of Vienna, Vienna, Austria Amide activation: an emerging tool for chemoselective synthesis Let them stand out of the crowd – Amide activation enables the chemoselective modification of a large variety of molecules while leaving many other functional groups untouched, making it attractive for the synthesis of sophisticated targets. This issue features a review on this emerging field and its application in total synthesis. See Nuno Maulide et al., Chem. Soc. Rev., 2018, 47, 7899. rsc.li/chem-soc-rev Registered charity number: 207890 Chem Soc Rev View Article Online REVIEW ARTICLE View Journal | View Issue Amide activation: an emerging tool for chemoselective synthesis Cite this: Chem. Soc. Rev., 2018, 47,7899 Daniel Kaiser, Adriano Bauer, Miran Lemmerer and Nuno Maulide * It is textbook knowledge that carboxamides benefit from increased stabilisation of the electrophilic carbonyl carbon when compared to other carbonyl and carboxyl derivatives. This results in a considerably reduced reactivity towards nucleophiles. Accordingly, a perception has been developed of amides as significantly less useful functional handles than their ester and acid chloride counterparts. Received 27th April 2018 However, a significant body of research on the selective activation of amides to achieve powerful DOI: 10.1039/c8cs00335a transformations under mild conditions has emerged over the past decades. This review article aims at placing electrophilic amide activation in both a historical context and in that of natural product rsc.li/chem-soc-rev synthesis, highlighting the synthetic applications and the potential of this approach. Creative Commons Attribution 3.0 Unported Licence. -

United States Patent Office Patented Nov
3,221,026 United States Patent Office Patented Nov. 30, 1965 2 3,221,026 prepared by reaction of a dicyanoketene acetal of the SALTS OF 1,1-DCYANO-2,2,2-TRIALKOXY formula ETHANES Owen W. Webster, Wilmington, Del, assignor to E. I. du Pont de Nemours and Company, Wilmington, Del., a corporation of Delaware No Drawing. Filed Feb. 13, 1962, Ser. No. 172,875 wherein R2 and R3 have the meanings defined above in the 2 Claims. (C. 260-340.9) general formula for the products of this invention, with This invention relates to salts of polycyano compounds, one molar equivalent of an alkali metal alkoxide of an and more particularly, to salts of polycyanopolyalkoxy alcohol having 1-8 carbon atoms at a temperature below ethanes and a process for their preparation. 10° C., and preferably at a temperature between 0 and The salts are derivatives of tetracyanoethylene which -80° C., in the presence of an inert reaction medium, is a very reactive compound that has received considerable e.g., an excess of the alcohol from which the alkoxide is study during the last few years. A large number of new 5 derived, or an ether such as diethyl ether, dioxane, tetra and valuable compounds have been prepared from it, and hydrofuran, ethylene glycol dimethyl ether and the like. now a new class of polycyano compounds is provided by As in the case of the reaction starting with tetracyano the present invention. ethylene, the reaction mixture in this case should also The novel compounds of this invention are salts of the be anhydrous to obtain the best results. -

Synthesis of Novel Single-Source Precursors for CVD of Mixed-Metal Tungsten Oxide
Synthesis of novel single-source precursors for CVD of mixed-metal tungsten oxide Hamid Choujaa A thesis submitted for the degree of Doctor of Philosophy University of Bath Department of Chemistry March 2008 COPYRIGHT Attention is drawn to the fact that copyright of this thesis rests with its author. This copy of the thesis has been supplied on condition that anyone who consults it is understood to recognize that its copyright rests with its author and that no quotation the thesis and no information derived from it may be published without the prior written consent of the author. This thesis may be made available for consultation within the University Library and may be photocopied or lent to other libraries for the purposes of consultation. TABLE OF CONTENTS Abstract ....................................................................................................................................... i Acknowledgements .................................................................................................................... iii Abbreviations and Acronyms .................................................................................................... iv 1. INTRODUCTION .................................................................................................................. 1 1.1 Generality about tungsten(VI) oxide ............................................................................. 1 1.1.1 The different lattice structures of tungsten oxide ........................................... 1 1.1.2 Electronic and