Risk Evaluation for 1-Bromopropane (N-Propyl Bromide) CASRN: 106-94-5
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Radiation Forces on Small Particles in the Solar System T
1CARUS 40, 1-48 (1979) Radiation Forces on Small Particles in the Solar System t JOSEPH A. BURNS Cornell University, 2 Ithaca, New York 14853 and NASA-Ames Research Center PHILIPPE L. LAMY CNRS-LAS, Marseille, France 13012 AND STEVEN SOTER Cornell University, Ithaca, New York 14853 Received May 12, 1978; revised May 2, 1979 We present a new and more accurate expression for the radiation pressure and Poynting- Robertson drag forces; it is more complete than previous ones, which considered only perfectly absorbing particles or artificial scattering laws. Using a simple heuristic derivation, the equation of motion for a particle of mass m and geometrical cross section A, moving with velocity v through a radiation field of energy flux density S, is found to be (to terms of order v/c) mi, = (SA/c)Qpr[(1 - i'/c)S - v/c], where S is a unit vector in the direction of the incident radiation,/" is the particle's radial velocity, and c is the speed of light; the radiation pressure efficiency factor Qpr ~ Qabs + Q~a(l - (cos a)), where Qabs and Q~c~ are the efficiency factors for absorption and scattering, and (cos a) accounts for the asymmetry of the scattered radiation. This result is confirmed by a new formal derivation applying special relativistic transformations for the incoming and outgoing energy and momentum as seen in the particle and solar frames of reference. Qpr is evaluated from Mie theory for small spherical particles with measured optical properties, irradiated by the actual solar spectrum. Of the eight materials studied, only for iron, magnetite, and graphite grains does the radiation pressure force exceed gravity and then just for sizes around 0. -

Exam 1 (February 23, 2004) ID# ______
Chemistry 211 Name ___________________________ Exam 1 (February 23, 2004) ID# ___________________________ 10 1. You desire to synthesize 3-ethyl-3-pentanol starting with an ester. (i) What would be the name of the ester, and what is the name for the Grignard reagent (e.g., methyl magnesium bromide)? (ii) For the carbons shown in the product, show plausible hydrocarbons that you could start with to produce the ester and the Grignard reagent (as in a retrosynthesis). 12 2. (i) Show the step-by-step process required to produce propyllithium, which requires a free radical reaction mechanism, . (ii) Show the complete reaction mechanism for reaction between propyllithium and the correct ketone to produce 3-propyl-3-pentanol. (iii) Propose a possible reaction mechanism by which dipropyl cuprate (Cu+ with two propyl groups attached) could react with ethyl bromide to produce a new hydrocarbon. (This is a thinking exercise! So, think! () 8 3. As mentioned in the text, diethyl ether, pentane, and 1-butanol have similar molar masses, but different physical properties. Boiling points are 35oC, 36oC, and 117oC, respectively. Their respective solubilities in water are 7.5g/100mL, insoluble, and 9g/100mL. (i) Draw structures for each of these compounds. (ii) Justify the observed boiling points and their solubilities. 16 4. Draw structures of the following compounds 2,3-heptanediol isopropyllithium benzylmagnesium bromide benzoic acid benzaldehylde dimethyl sulfide t-butyl methanoate dibutyl ketone 12 5. Alcohols can be oxidized to produce other compounds, and can be produced by reduction. For the reactions shown below, show the structure for the expected product (if reaction does not occur, state: No Reaction) when treated with the indicated oxidizing or reducing agents. -

Chemistry for S5 Students Short Notes & Questions with Answers & And
Chemistry for S5 students Short notes & questions with answers & and other questions to help S5 Students to revise Important Concepts: Chemical Reactions of Alkyl Halides The reaction can be broadly classified in two categories: (a) Nucleophilic substitution (b) Elimination reactions Nucleophilic substitution reactions: In this reaction a nucleophile, which is rich in electrons, attacks partial positive charge on the carbon atom bonded to halogen to replace the leaving group. Nucleophilic reactions proceed by two different mechanism: (a) Substitution nucleophilic bimolecular (SN2) (b) Substitution nucleophilic unirnolecular (SN1) The reaction follows second order kinetics No intermediate is formed. It usually requires a strong nucleophile. The order of reactivity followed as: Primary halide > Secondary halide > Tertiary halide It is carried out in polar protic solvents (water, Alcohol, acetic acid etc.). These reactions occur in two steps as shown above The order of leaving ability is: F- < Cl- < Br- < I- The order of reactivity is as shown below: Difference between E1 and E2 reaction mechanism: Attributes E1 E2 Rate law Depend on the concentration of Depends on the concentration of both substrate substrate and base Barrier Formation of carbocation None 3o>2o>> 1o Base Does not require strong base Requires strong base Stereochemistry Does not require stereochemistry Leaving group must be anti to hydrogen removed Some solved questions are given below: Question 1:Which is the correct increasing order of boiling points of the following compounds? 1-bromoethane, 1-bromopropane, 1-bromobutane, Bromobenzene (a) Bromobenzene < 1-bromobutane < 1-bromopropane < 1-bromoethane (b) Bromobenzene < 1-bromothane < 1-bromopropane < 1-bromobutane (c) 1-bromopropane < 1-bromobutane < 1-bromoethane < Bromobenzene (d) 1-bromoethane < 1-bromopropane < 1-bromobutane < Bromobenzene Solution 1: Boiling point increases with increase in size of hydrocarbon part for the same haloalkanes. -

Planetary Defence Activities Beyond NASA and ESA
Planetary Defence Activities Beyond NASA and ESA Brent W. Barbee 1. Introduction The collision of a significant asteroid or comet with Earth represents a singular natural disaster for a myriad of reasons, including: its extraterrestrial origin; the fact that it is perhaps the only natural disaster that is preventable in many cases, given sufficient preparation and warning; its scope, which ranges from damaging a city to an extinction-level event; and the duality of asteroids and comets themselves---they are grave potential threats, but are also tantalising scientific clues to our ancient past and resources with which we may one day build a prosperous spacefaring future. Accordingly, the problems of developing the means to interact with asteroids and comets for purposes of defence, scientific study, exploration, and resource utilisation have grown in importance over the past several decades. Since the 1980s, more and more asteroids and comets (especially the former) have been discovered, radically changing our picture of the solar system. At the beginning of the year 1980, approximately 9,000 asteroids were known to exist. By the beginning of 2001, that number had risen to approximately 125,000 thanks to the Earth-based telescopic survey efforts of the era, particularly the emergence of modern automated telescopic search systems, pioneered by the Massachusetts Institute of Technology’s (MIT’s) LINEAR system in the mid-to-late 1990s.1 Today, in late 2019, about 840,000 asteroids have been discovered,2 with more and more being found every week, month, and year. Of those, approximately 21,400 are categorised as near-Earth asteroids (NEAs), 2,000 of which are categorised as Potentially Hazardous Asteroids (PHAs)3 and 2,749 of which are categorised as potentially accessible.4 The hazards posed to us by asteroids affect people everywhere around the world. -

Asteroid Retrieval Feasibility Study
Asteroid Retrieval Feasibility Study 2 April 2012 Prepared for the: Keck Institute for Space Studies California Institute of Technology Jet Propulsion Laboratory Pasadena, California 1 2 Authors and Study Participants NAME Organization E-Mail Signature John Brophy Co-Leader / NASA JPL / Caltech [email protected] Fred Culick Co-Leader / Caltech [email protected] Co -Leader / The Planetary Louis Friedman [email protected] Society Carlton Allen NASA JSC [email protected] David Baughman Naval Postgraduate School [email protected] NASA ARC/Carnegie Mellon Julie Bellerose [email protected] University Bruce Betts The Planetary Society [email protected] Mike Brown Caltech [email protected] Michael Busch UCLA [email protected] John Casani NASA JPL [email protected] Marcello Coradini ESA [email protected] John Dankanich NASA GRC [email protected] Paul Dimotakis Caltech [email protected] Harvard -Smithsonian Center for Martin Elvis [email protected] Astrophysics Ian Garrick-Bethel UCSC [email protected] Bob Gershman NASA JPL [email protected] Florida Institute for Human and Tom Jones [email protected] Machine Cognition Damon Landau NASA JPL [email protected] Chris Lewicki Arkyd Astronautics [email protected] John Lewis University of Arizona [email protected] Pedro Llanos USC [email protected] Mark Lupisella NASA GSFC [email protected] Dan Mazanek NASA LaRC [email protected] Prakhar Mehrotra Caltech [email protected] -

Constraints on a Potential Aerial Biosphere on Venus: I. Cosmic Rays ⇑ Lewis R
Icarus 257 (2015) 396–405 Contents lists available at ScienceDirect Icarus journal homepage: www.elsevier.com/locate/icarus Constraints on a potential aerial biosphere on Venus: I. Cosmic rays ⇑ Lewis R. Dartnell a, , Tom Andre Nordheim b,c, Manish R. Patel d,e, Jonathon P. Mason d, Andrew J. Coates b,c, Geraint H. Jones b,c a Space Research Centre, Department of Physics and Astronomy, University of Leicester, Leicester LE1 7RH, UK b Mullard Space Science Laboratory, University College London, Dorking, Surrey RH5 6NT, UK c Centre for Planetary Sciences at UCL/Birkbeck, University College London, Gower Street, London WC1E 6BT, UK d Department of Physical Sciences, The Open University, Milton Keynes, UK e Space Science and Technology Department, STFC Rutherford Appleton Laboratory, Chilton, Oxfordshire OX11 0QX, UK article info abstract Article history: While the present-day surface of Venus is certainly incompatible with terrestrial biology, the planet may Received 31 October 2014 have possessed oceans in the past and provided conditions suitable for the origin of life. Venusian life Revised 10 April 2015 may persist today high in the atmosphere where the temperature and pH regime is tolerable to terrestrial Accepted 7 May 2015 extremophile microbes: an aerial habitable zone. Here we argue that on the basis of the combined Available online 15 May 2015 biological hazard of high temperature and high acidity this habitable zone lies between 51 km (65 °C) and 62 km (À20 °C) altitude. Compared to Earth, this potential venusian biosphere may be exposed to Keywords: substantially more comic ionising radiation: Venus has no protective magnetic field, orbits closer to Astrobiology the Sun, and the entire habitable region lies high in the atmosphere – if this narrow band is sterilised Cosmic rays Exobiology there is no reservoir of deeper life that can recolonise afterwards. -

Problems with Panspermia Or Extraterrestrial Origin of Life Scenarios As Found on the IDEA Center Website At
Problems with Panspermia or Extraterrestrial Origin of Life Scenarios As found on the IDEA Center website at http://www.ideacenter.org Panspermia is the theory that microorganisms or biochemical compounds from outer space are responsible for originating life on Earth and possibly in other parts of the universe where suitable atmospheric conditions exist. Essentially, it is a hypothesis which states that life on earth came from outer space. It has been popularized in countless science fiction works, however some scientists, including the discoverer of the structure of DNA, Francis Crick, have advocated panspermia. There are generally about 3 different "flavors" of panspermia: 1. Naturalistic Panspermia where life evolves on another planet, and naturally gets ejected off the planet and come to rest on earth. 2. Directed Panspermia where intelligent life on other planets intentionally seeded other planets with their own form of life. 3. Intelligent Design Panspermia, where intelligent beings from another planet came to earth and designed life here. Each type of panspermia will be briefly discussed below: 1. Naturalistic Panspermia where life evolves on another planet, and naturally gets ejected off the planet and come to rest on earth. Naturalistic panspermia has gained popularity because some have recognized that life on earth appears very soon after the origin of conditions which would allow it to exist. Many scientists believed that if life had originated on earth it would have taken many hundreds of millions, or even billions of years to do so. Life appears perhaps less than 200 million after the origin of conditions at which life could exist. -
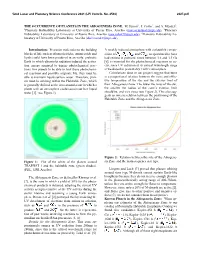
The Occurrence of Planets in the Abiogenesis Zone
52nd Lunar and Planetary Science Conference 2021 (LPI Contrib. No. 2548) 2327.pdf THE OCCURRENCE OF PLANETS IN THE ABIOGENESIS ZONE. M. Jusino1 , J. Colón2 , and A. Méndez3, 1Planetary Habitability Laboratory at University of Puerto Rico, Arecibo ([email protected]), 2Planetary Habitability Laboratory at University of Puerto Rico, Arecibo ([email protected]), 3Planetary Habitability La- boratory at University of Puerto Rico, Arecibo ([email protected]) Introduction: Precursor molecules to the building A weakly reduced atmosphere with a plausible compo- blocks of life such as ribonucleotides, amino acids and sition of , , , and , as speculated to have lipids could have been produced in an early, prebiotic had existed in prebiotic times between 3.8 and 3.5 Ga Earth in which ultraviolet radiation induced the activa- [6], is essential for the photochemical reactions to oc- tion energy required to trigger photochemical reac- cur, since UV radiation at its critical wavelength range tions. For planets to be able to host these photochemi- is weakened in present-day Earth’s atmosphere. cal reactions and possibly originate life, they must be Calculations done in our project suggest that there able to maintain liquid surface water. Therefore, plan- is a proportional relation between the mass and effec- ets must be orbiting within the Habitable Zone, which tive temperature of the star and the exterior limit of is generally defined as the area around a star in which a their Abiogenesis Zone. The lower the mass of the star, planet with an atmosphere could sustain surface liquid the smaller the radius of the zone’s exterior limit water [1]. -

Carl Sagan: the People’S Astronomer by David Morrison, NASA, Ames Research Center
Carl Sagan: The People’s Astronomer by David Morrison, NASA, Ames Research Center Introduction Carl Sagan was the world’s best known scientist in the late 20th century, serving as our guide to the planets during the golden age of solar system exploration. He was both a visionary and a committed defender of rational scientific thinking. For a time, he transcended the usual categories of academics to become a true celebrity. His life illustrates both the advantages (wealth, fame, access to the seats of power) and burdens (loss of privacy, stress, criticism from academic colleagues) this status implies. Sagan was propelled on his academic and public careers by a wealth of talent, a large share of good luck, and an intensely focused drive to succeed. His lifelong quest was to understand the universe, especially our planetary system, and to communicate the thrill of scientific discovery to others. A natural teacher, he loved to explain things and never made a questioner feel stupid for asking. Although Sagan had broad intellectual interests, his pursuit of his career left little time for other activities: he did not play golf or follow sports, take up painting or cooking or photography, sing or play a musical instrument, join a church or synagogue, or watch much television or movies. His first two wives complained that he devoted insufficient time to his marriage or his children (1). It is perhaps a matter of personal taste whether we attribute this drive to personal ego or a genuine commitment to educate and inspire people about science. Undoubtedly there were elements of both motivations present. -

Chapter 18 Ethers and Epoxides; Thiols and Sulfides Ethers
Chapter 18 Ethers and Epoxides; Thiols and Sulfides Ethers • Ethers (R–O–R’): – Organic derivatives of water, having two organic groups bonded to the same oxygen atom © 2016 Cengage Learning 2 NAMES AND PROPERTIES OF ETHERS 3 Nomenclature: Common Names • Simple ethers are named by identifying two organic substituents and adding the word ether – Name the groups in alphabetical order – Symmetrical: Use dialkyl or just alkyl © 2016 Cengage Learning 4 Nomenclature: IUPAC Names • The more complex alkyl group is the parent name • The group with the oxygen becomes an alkoxy group © 2016 Cengage Learning 5 Nomenclature: Cyclic Ethers (Heterocycles) • Heterocyclic: Oxygen is part of the ring. O • Epoxides (oxiranes) H2C CH2 O • Oxetanes • Furans (Oxolanes) O O • Pyrans (Oxanes) O O O • Dioxanes O © 2013 Pearson Education, Inc. 6 Epoxide Nomenclature • Name the starting alkene and add “oxide” © 2013 Pearson Education, Inc. 7 Epoxide Nomenclature • The oxygen can be treated as a substituent (epoxy) on the compound • Use numbers to specify position • Oxygen is 1, the carbons are 2 and 3 • Substituents are named in alphabetical order © 2013 Pearson Education, Inc. 8 Properties of Ethers • Possess nearly the same geometry as water – Oxygen atom is sp3-hybridized – Bond angles of R–O–R bonds are approximately tetrahedral • Polar C—O bonds © 2013 Pearson Education, Inc. 9 Properties of Ethers: Hydrogen Bond • Hydrogen bond is a attractive interaction between an electronegative atom and a hydrogen atom bonded to another electronegative atom • Ethers cannot hydrogen bond with other ether molecules, so they have a lower boiling point than alcohols • Ether molecules can hydrogen bond with water and alcohol molecules • They are hydrogen bond acceptors © 2013 Pearson Education, Inc. -

Synthesis and Reactivity of Cyclopentadienyl Based Organometallic Compounds and Their Electrochemical and Biological Properties
Synthesis and reactivity of cyclopentadienyl based organometallic compounds and their electrochemical and biological properties Sasmita Mishra Department of Chemistry National Institute of Technology Rourkela Synthesis and reactivity of cyclopentadienyl based organometallic compounds and their electrochemical and biological properties Dissertation submitted to the National Institute of Technology Rourkela In partial fulfillment of the requirements of the degree of Doctor of Philosophy in Chemistry by Sasmita Mishra (Roll Number: 511CY604) Under the supervision of Prof. Saurav Chatterjee February, 2017 Department of Chemistry National Institute of Technology Rourkela Department of Chemistry National Institute of Technology Rourkela Certificate of Examination Roll Number: 511CY604 Name: Sasmita Mishra Title of Dissertation: ''Synthesis and reactivity of cyclopentadienyl based organometallic compounds and their electrochemical and biological properties We the below signed, after checking the dissertation mentioned above and the official record book(s) of the student, hereby state our approval of the dissertation submitted in partial fulfillment of the requirements of the degree of Doctor of Philosophy in Chemistry at National Institute of Technology Rourkela. We are satisfied with the volume, quality, correctness, and originality of the work. --------------------------- Prof. Saurav Chatterjee Principal Supervisor --------------------------- --------------------------- Prof. A. Sahoo. Prof. G. Hota Member (DSC) Member (DSC) --------------------------- -

Francis Crick, Cross-Worlds Influencer: a Narrative Model to Historicize Big Bioscience
King’s Research Portal DOI: 10.1016/j.shpsc.2015.08.003 Document Version Publisher's PDF, also known as Version of record Link to publication record in King's Research Portal Citation for published version (APA): Aicardi, C. (2016). Francis Crick, cross-worlds influencer: A narrative model to historicize big bioscience. Studies in History and Philosophy of Science Part C: Studies in History and Philosophy of Biological and Biomedical Sciences, 55, 83-95. https://doi.org/10.1016/j.shpsc.2015.08.003 Citing this paper Please note that where the full-text provided on King's Research Portal is the Author Accepted Manuscript or Post-Print version this may differ from the final Published version. If citing, it is advised that you check and use the publisher's definitive version for pagination, volume/issue, and date of publication details. And where the final published version is provided on the Research Portal, if citing you are again advised to check the publisher's website for any subsequent corrections. General rights Copyright and moral rights for the publications made accessible in the Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognize and abide by the legal requirements associated with these rights. •Users may download and print one copy of any publication from the Research Portal for the purpose of private study or research. •You may not further distribute the material or use it for any profit-making activity or commercial gain •You may freely distribute the URL identifying the publication in the Research Portal Take down policy If you believe that this document breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim.