Exam 1 (February 23, 2004) ID# ______
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemistry for S5 Students Short Notes & Questions with Answers & And
Chemistry for S5 students Short notes & questions with answers & and other questions to help S5 Students to revise Important Concepts: Chemical Reactions of Alkyl Halides The reaction can be broadly classified in two categories: (a) Nucleophilic substitution (b) Elimination reactions Nucleophilic substitution reactions: In this reaction a nucleophile, which is rich in electrons, attacks partial positive charge on the carbon atom bonded to halogen to replace the leaving group. Nucleophilic reactions proceed by two different mechanism: (a) Substitution nucleophilic bimolecular (SN2) (b) Substitution nucleophilic unirnolecular (SN1) The reaction follows second order kinetics No intermediate is formed. It usually requires a strong nucleophile. The order of reactivity followed as: Primary halide > Secondary halide > Tertiary halide It is carried out in polar protic solvents (water, Alcohol, acetic acid etc.). These reactions occur in two steps as shown above The order of leaving ability is: F- < Cl- < Br- < I- The order of reactivity is as shown below: Difference between E1 and E2 reaction mechanism: Attributes E1 E2 Rate law Depend on the concentration of Depends on the concentration of both substrate substrate and base Barrier Formation of carbocation None 3o>2o>> 1o Base Does not require strong base Requires strong base Stereochemistry Does not require stereochemistry Leaving group must be anti to hydrogen removed Some solved questions are given below: Question 1:Which is the correct increasing order of boiling points of the following compounds? 1-bromoethane, 1-bromopropane, 1-bromobutane, Bromobenzene (a) Bromobenzene < 1-bromobutane < 1-bromopropane < 1-bromoethane (b) Bromobenzene < 1-bromothane < 1-bromopropane < 1-bromobutane (c) 1-bromopropane < 1-bromobutane < 1-bromoethane < Bromobenzene (d) 1-bromoethane < 1-bromopropane < 1-bromobutane < Bromobenzene Solution 1: Boiling point increases with increase in size of hydrocarbon part for the same haloalkanes. -

Grignard Reaction: Synthesis of Triphenylmethanol
*NOTE: Grignard reactions are very moisture sensitive, so all the glassware in the reaction (excluding the work-up) should be dried in an oven with a temperature of > 100oC overnight. The following items require oven drying. They should be placed in a 150mL beaker, all labeled with a permanent marker. 1. 5mL conical vial (AKA: Distillation receiver). 2. Magnetic spin vane. 3. Claisen head. 4. Three Pasteur pipettes. 5. Two 1-dram vials (Caps EXCLUDED). 6. One 2-dram vial (Caps EXCLUDED). 7. Glass stirring rod 8. Adaptor (19/22.14/20) Grignard Reaction: Synthesis of Triphenylmethanol Pre-Lab: In the “equations” section, besides the main equations, also: 1) draw the equation for the production of the byproduct, Biphenyl. 2) what other byproduct might occur in the reaction? Why? In the “observation” section, draw data tables in the corresponding places, each with 2 columns -- one for “prediction” (by answering the following questions) and one for actual drops or observation. 1) How many drops of bromobenzene should you add? 2) How many drops of ether will you add to flask 2? 3) 100 µL is approximately how many drops? 4) What are the four signs of a chemical reaction? (Think back to Chem. 110) 5) How do the signs of a chemical reaction apply to this lab? The Grignard reaction is a useful synthetic procedure for forming new carbon- carbon bonds. This organometallic chemical reaction involves alkyl- or aryl-magnesium halides, known as Grignard 1 reagents. Grignard reagents are formed via the action of an alkyl or aryl halide on magnesium metal. -

Organic Chemistry Laboratory II Preparation of Triphenylmethanol (Grignard Reaction) Experiment Procedure (Printable Pdf Format)
grigdes http://www.organicchem.org/oc2web/lab/exp/grig/grigdesbenzophenone... Organic Chemistry II | Lecture | Laboratory Organic Chemistry Laboratory II Preparation of Triphenylmethanol (Grignard Reaction) Experiment Procedure (Printable pdf format) Introduction In this two-week experiment, triphenylmethanol will be synthesized through a Grignard reaction. Students will work individually to prepare the Grignard reagent by reacting bromobenzene with solid magnesium in diethylether. The prepared Grignard reagent, phenylmagnesium bromide, will then be combined with bezophenone to form the desired triphenylmethanol product. Procedure NOTE: All glassware must be extremely dry! Place glassware in the drying oven for at least 10 minutes before beginning the reaction. Preparation of the Grignard Reagent Add ~0.5g of magnesium turnings and a magnetic stir bar to a dry 100 ml round-bottomed flask, and place the flask in the drying oven for 30 minutes. Remove the flask from the oven, clamp it to a ring stand, insert a reflux condenser, fit the flask into a heating mantle and immediately attach a drying tube to the top of the reflux condenser. Position the flask in the heating mantle on a stirrer/hotplate. Set the reaction up on the side of the hood near to the faucet labeled for distillation. Allow the reaction flask to cool to room temperature completely before proceeding. While the flask is cooling, place a 150ml beaker into the drying oven. While the flask is cooling, add 3.5 g of dry bromobenzene to a dry 50 mL Erlenmeyer flask and dissolve it in 5.0 mL of anhydrous diethyl ether. Use a glass powder funnel to dispense the bromobenze and ether to the Erlenmyer flask. -

Risk Evaluation for 1-Bromopropane (N-Propyl Bromide) CASRN: 106-94-5
United States EPA Document #740-R1-8013 Environmental Protection Agency August 2020 Office of Chemical Safety and Pollution Prevention Risk Evaluation for 1-Bromopropane (n-Propyl Bromide) CASRN: 106-94-5 August 2020 TABLE OF CONTENTS TABLE OF CONTENTS ............................................................................................................. 2 LIST OF TABLES ...................................................................................................................... 10 LIST OF APPENDIX TABLES ................................................................................................ 17 LIST OF FIGURES .................................................................................................................... 17 LIST OF APPENDIX FIGURES .............................................................................................. 19 LIST OF EQUATIONS .............................................................................................................. 19 LIST OF APPENDIX EQUATIONS ........................................................................................ 20 ACKNOWLEDGEMENTS ....................................................................................................... 21 ABBREVIATIONS ..................................................................................................................... 22 EXECUTIVE SUMMARY ........................................................................................................ 30 1 INTRODUCTION................................................................................................................... -
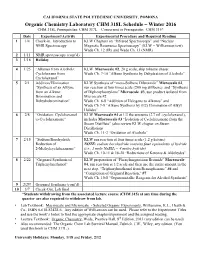
318 Lab Schedule
CALIFORNIA STATE POLYTECHNIC UNIVERSITY, POMONA Organic Chemistry Laboratory CHM 318L Schedule – Winter 2016 CHM 318L Prerequisites: CHM 317L Concurrent or Prerequisite: CHM 315* Date Experiment/Activity Experimental Procedure and Required Reading 1 1/4 Check-in. Introduction to KLW Chapters on “Infrared Spectroscopy” and “Nuclear NMR Spectroscopy Magnetic Resonance Spectroscopy” (KLW = Williamson text) Wade Ch. 12 (IR) and Wade Ch. 13 (NMR) 2 1/11 NMR spectroscopy (cont’d) 3 1/18 Holiday 4 1/25 “Alkenes from Alcohols: KLW Macroscale #2, 20 g scale, skip toluene chaser Cyclohexene from Wade Ch. 7-10 “Alkene Synthesis by Dehydration of Alcohols” Cyclohexanol” 5 2/1 Addition/Elimination KLW Synthesis of “meso-Stilbene Dibromide” Microscale #2, “Synthesis of an Alkyne run reaction at four times scale (200 mg stillbene); and “Synthesis from an Alkene: of Diphenylacetylene” Microscale #3, use product isolated from Bromination and Microscale #2 Dehydrobromination” Wade Ch. 8-8 “Addition of Halogens to Alkenes” and Wade Ch 7-9 “Alkene Synthesis by (E2) Elimination of Alkyl Halides” 6 2/8 “Oxidation: Cyclohexanol KLW Macroscale #4 at 1/3 the amounts (2.7 mL cyclohexanol), to Cyclohexanone” includes Macroscale #3 “Isolation of Cyclohexanone from the Steam Distillate” (also review KLW chapter on Steam Distillation) Wade Ch. 11-2 “Oxidation of Alcohols” 7 2/15 “Sodium Borohydride KLW run reaction at four times scale (1.2 g ketone) Reduction of NOTE: sodium borohydride contains four equivalents of hydride 2-Methylcyclohexanone” (i.e., 1 mole NaBH4 = 4 moles hydride) Wade Ch. 10-11 & 18-20 “Reductions of Ketones & Aldehydes” 8 2/22 “Grignard Synthesis of KLW preparation of “Phenylmagnesium Bromide” Macroscale Triphenylmethanol” #4, run reaction at 1/2 scale and then use the entire amount in the next step, “Triphenylmethanol from Benzophenone” #6 and “Completion of Grignard Reaction,” #7 Wade Ch. -

Revised Group Additivity Values for Enthalpies of Formation (At 298 K) of Carbon– Hydrogen and Carbon–Hydrogen–Oxygen Compounds
Revised Group Additivity Values for Enthalpies of Formation (at 298 K) of Carbon– Hydrogen and Carbon–Hydrogen–Oxygen Compounds Cite as: Journal of Physical and Chemical Reference Data 25, 1411 (1996); https://doi.org/10.1063/1.555988 Submitted: 17 January 1996 . Published Online: 15 October 2009 N. Cohen ARTICLES YOU MAY BE INTERESTED IN Additivity Rules for the Estimation of Molecular Properties. Thermodynamic Properties The Journal of Chemical Physics 29, 546 (1958); https://doi.org/10.1063/1.1744539 Critical Evaluation of Thermochemical Properties of C1–C4 Species: Updated Group- Contributions to Estimate Thermochemical Properties Journal of Physical and Chemical Reference Data 44, 013101 (2015); https:// doi.org/10.1063/1.4902535 Estimation of the Thermodynamic Properties of Hydrocarbons at 298.15 K Journal of Physical and Chemical Reference Data 17, 1637 (1988); https:// doi.org/10.1063/1.555814 Journal of Physical and Chemical Reference Data 25, 1411 (1996); https://doi.org/10.1063/1.555988 25, 1411 © 1996 American Institute of Physics for the National Institute of Standards and Technology. Revised Group Additivity Values for Enthalpies of Formation (at 298 K) of Carbon-Hydrogen and Carbon-Hydrogen-Oxygen Compounds N. Cohen Thermochemical Kinetics Research, 6507 SE 31st Avenue, Portland, Oregon 97202-8627 Received January 17, 1996; revised manuscript received September 4, 1996 A program has been undertaken for the evaluation and revision of group additivity values (GAVs) necessary for predicting, by means of Benson's group additivity method, thermochemical properties of organic molecules. This review reports on the portion of that program dealing with GAVs for enthalpies of formation at 298.15 K (hereinafter abbreviated as 298 K) for carbon-hydrogen and carbon-hydrogen-oxygen compounds. -

Chapter 18 Ethers and Epoxides; Thiols and Sulfides Ethers
Chapter 18 Ethers and Epoxides; Thiols and Sulfides Ethers • Ethers (R–O–R’): – Organic derivatives of water, having two organic groups bonded to the same oxygen atom © 2016 Cengage Learning 2 NAMES AND PROPERTIES OF ETHERS 3 Nomenclature: Common Names • Simple ethers are named by identifying two organic substituents and adding the word ether – Name the groups in alphabetical order – Symmetrical: Use dialkyl or just alkyl © 2016 Cengage Learning 4 Nomenclature: IUPAC Names • The more complex alkyl group is the parent name • The group with the oxygen becomes an alkoxy group © 2016 Cengage Learning 5 Nomenclature: Cyclic Ethers (Heterocycles) • Heterocyclic: Oxygen is part of the ring. O • Epoxides (oxiranes) H2C CH2 O • Oxetanes • Furans (Oxolanes) O O • Pyrans (Oxanes) O O O • Dioxanes O © 2013 Pearson Education, Inc. 6 Epoxide Nomenclature • Name the starting alkene and add “oxide” © 2013 Pearson Education, Inc. 7 Epoxide Nomenclature • The oxygen can be treated as a substituent (epoxy) on the compound • Use numbers to specify position • Oxygen is 1, the carbons are 2 and 3 • Substituents are named in alphabetical order © 2013 Pearson Education, Inc. 8 Properties of Ethers • Possess nearly the same geometry as water – Oxygen atom is sp3-hybridized – Bond angles of R–O–R bonds are approximately tetrahedral • Polar C—O bonds © 2013 Pearson Education, Inc. 9 Properties of Ethers: Hydrogen Bond • Hydrogen bond is a attractive interaction between an electronegative atom and a hydrogen atom bonded to another electronegative atom • Ethers cannot hydrogen bond with other ether molecules, so they have a lower boiling point than alcohols • Ether molecules can hydrogen bond with water and alcohol molecules • They are hydrogen bond acceptors © 2013 Pearson Education, Inc. -

Grignard Synthesis of Triphenylmethanol Reactions That Form Carbon-Carbon Bonds Are Among the Most Useful to the Synthetic Organic Chemist
1 Experiment 12: Grignard Synthesis of Triphenylmethanol Reactions that form carbon-carbon bonds are among the most useful to the synthetic organic chemist. In 1912, Victor Grignard received the Nobel prize in chemistry for his discovery of a new series of reactions that result in the formation of a carbon-carbon bond. A Grignard synthesis first involves the preparation of an organomagnesium reagent via the reaction of an alkyl bromide with magnesium metal: δ– δ+ R Br + Mg R MgBr The resulting “Grignard reagent” acts as both a good nucleophile and a strong base. Its nucleophilic character allows it to react with the electrophilic carbon in a carbonyl group, thus forming the carbon-carbon bond. Its basic property means that it will react with acidic compounds, such as carboxylic acids, phenols, thiols and even alcohols and water; therefore, reaction conditions must be free from acids and strictly anhydrous. Grignard reagents will also react with oxygen to form hydroperoxides, thus they are highly unstable when exposed to the atmosphere and are generally not isolated from solution. For a variety of reasons, anhydrous diethyl ether is the solvent of choice for carrying out a Grignard synthesis. Vapors from the highly volatile solvent help to prevent oxygen from reaching the reaction solution. In addition, evidence suggests that the ether molecules actually coordinate with and help stabilize the Grignard reagent: Et Et O R Mg Br O Et Et The magnesium metal used in the synthesis contains a layer of oxide on the surface that prevents it from reacting with the alkyl bromide. The pieces of metal must be gently scratched while in the ether solution to expose fresh surface area so that the reaction can commence. -

The Effects of Stoichiometry and Starting Material on the Product Identity and Yield in Grignard Addition Reactions
Supplementary information for Comprehensive Organic Chemistry Experiments for the Laboratory Classroom © The Royal Society of Chemistry 2017 The effects of stoichiometry and starting material on the product identity and yield in Grignard addition reactions Supplementary Material This experiment has been performed both in the 150 person standard introductory organic chemistry laboratory (taught primarily by undergraduate teaching assistants in five sections of 30-40 students) and in a special introductory organic chemistry laboratory for freshman, taught to 23 students in sections of 7 and 16. This course is the first organic laboratory for these students but has been taught in the spring semester along with the second semester of organic lecture. The lab periods for this course are five hours long, and this experiment is typically performed in the latter half of the semester. This experiment is used to illustrate to students the importance of planning their time in lab; they must be out of the lab in the 5 hours allotted. While many students finish the experiment in this time, others plan to finish the following week along with a shorter experiment. Those who were running behind were encouraged to finish through the drying of their organic layer with MgSO4 and set up the distillation the following week. When doing this they should make sure that their organic layer is in a closed container to prevent evaporation of their product. Moisture Sensitive Conditions There are many diverse protocols for maintaining the anhydrous conditions required to successfully prepare and utilize Grignard reagents. We typically open a fresh can of anhydrous ether and dispense it directly, without additional drying in a still or air sensitive techniques for solvent transfers. -

Synthesis and Reactivity of Cyclopentadienyl Based Organometallic Compounds and Their Electrochemical and Biological Properties
Synthesis and reactivity of cyclopentadienyl based organometallic compounds and their electrochemical and biological properties Sasmita Mishra Department of Chemistry National Institute of Technology Rourkela Synthesis and reactivity of cyclopentadienyl based organometallic compounds and their electrochemical and biological properties Dissertation submitted to the National Institute of Technology Rourkela In partial fulfillment of the requirements of the degree of Doctor of Philosophy in Chemistry by Sasmita Mishra (Roll Number: 511CY604) Under the supervision of Prof. Saurav Chatterjee February, 2017 Department of Chemistry National Institute of Technology Rourkela Department of Chemistry National Institute of Technology Rourkela Certificate of Examination Roll Number: 511CY604 Name: Sasmita Mishra Title of Dissertation: ''Synthesis and reactivity of cyclopentadienyl based organometallic compounds and their electrochemical and biological properties We the below signed, after checking the dissertation mentioned above and the official record book(s) of the student, hereby state our approval of the dissertation submitted in partial fulfillment of the requirements of the degree of Doctor of Philosophy in Chemistry at National Institute of Technology Rourkela. We are satisfied with the volume, quality, correctness, and originality of the work. --------------------------- Prof. Saurav Chatterjee Principal Supervisor --------------------------- --------------------------- Prof. A. Sahoo. Prof. G. Hota Member (DSC) Member (DSC) --------------------------- -

CHM205 Chemicals by Experiment Tuesday, November 17, 2015 3:14:15 PM Experiment Title Chemical Name Concentration Acetaminophen Synthesis Acetic Anhydride Liquid
CHM205 Chemicals by Experiment Tuesday, November 17, 2015 3:14:15 PM Experiment Title Chemical Name Concentration Acetaminophen Synthesis Acetic anhydride liquid Acetaminophen Synthesis p-aminophenol solid Alcohols to Alkyl chlorides 2-pentanol liquid Alcohols to Alkyl chlorides Hydrochloric acid 12 M Alcohols to Alkyl chlorides Sodium carbonate solid Alcohols to Alkyl chlorides Hydrobromic acid 48% w/v Alcohols to Alkyl chlorides Sodium sulfate anhydrous solid Alcohols to Alkyl chlorides sec-phenethyl alcohol liquid Alcohols to Alkyl chlorides Benzyl alcohol liquid Alcohols to Alkyl chlorides t-butanol liquid Alcohols to Alkyl chlorides 1-pentanol liquid Alcohols to Alkyl chlorides Sodium carbonate 10% w/v Diels Alder Reaction 2,3-dimethyl-1,3-butadiene liquid Diels Alder Reaction Maleic anhydride solid Diels Alder Reaction Ethanol 95% Liquid Diels Alder Reaction Hexane liquid Diels Alder Reaction Cyclohexane liquid Diels Alder Reaction Calcium chloride solid Esterification methanol liquid Esterification Sodium carbonate 10% w/v Esterification 1-propanol liquid Esterification 1-butanol liquid Esterification trans-cinnamic acid solid Esterification Isoamyl alcohol liquid Esterification Isopropyl alcohol liquid Esterification Benzyl alcohol liquid Esterification Sulfuric acid conc. 18 M Esterification 1-pentanol liquid Esterification Isobutyl alcohol liquid Esterification Ethanol 95% liquid Page 1 of 3 Experiment Title Chemical Name Concentration Extraction of Beta Carotene Cyclohexane liquid Extraction of Beta Carotene Beta carotene UV -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride