Organic Chemistry, 5E (Bruice) Chapter 17: Carbonyl Compounds II
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Zr-Modified Zno for the Selective Oxidation of Cinnamaldehyde to Benzaldehyde
catalysts Article Zr-Modified ZnO for the Selective Oxidation of Cinnamaldehyde to Benzaldehyde Pengju Du 1, Tongming Su 1, Xuan Luo 1, Xinling Xie 1, Zuzeng Qin 1,* and Hongbing Ji 1,2 1 School of Chemistry and Chemical Engineering, Guangxi University, Nanning 530004, China 2 School of Chemistry, Sun Yat-sen University, Guangzhou 510275, China * Correspondence: [email protected]; Tel.: +86-771-323-3718 Received: 31 July 2019; Accepted: 21 August 2019; Published: 25 August 2019 Abstract: ZnO and Zr-modified ZnO were prepared using a precipitation method and used for the selective oxidation of cinnamaldehyde to benzaldehyde in the present study. The results showed that physicochemical properties of ZnO were significantly affected by the calcination temperature, and calcination of ZnO at 400 ◦C demonstrated the optimum catalytic activity for the selective oxidation of cinnamaldehyde to benzaldehyde. With 0.01 g ZnO calcined at 400 ◦C for 2 h as a catalyst, 8.0 g ethanol and 2.0 g cinnamaldehyde reacted at an oxygen pressure of 1.0 MPa and 70 ◦C for 60 min, resulting in benzaldehyde selectivity of 69.2% and cinnamaldehyde conversion of 16.1%. Zr was the optimal modifier for ZnO: when Zr-modified ZnO was used as the catalyst, benzaldehyde selectivity reached 86.2%, and cinnamaldehyde conversion was 17.6%. The X-ray diffractometer and N2 adsorption–desorption characterization indicated that doping with Zr could reduce the crystallite size of ZnO (101) and increase the specific surface area of the catalyst, which provided more active sites for the reaction. X-ray photoelectron spectrometer results showed that Zr-doping could exchange the electrons with ZnO and reduce the electron density in the outer layer of Zn, which would further affect benzaldehyde selectivity. -

Aldehydes and Ketones
12 Aldehydes and Ketones Ethanol from alcoholic beverages is first metabolized to acetaldehyde before being broken down further in the body. The reactivity of the carbonyl group of acetaldehyde allows it to bind to proteins in the body, the products of which lead to tissue damage and organ disease. Inset: A model of acetaldehyde. (Novastock/ Stock Connection/Glow Images) KEY QUESTIONS 12.1 What Are Aldehydes and Ketones? 12.8 What Is Keto–Enol Tautomerism? 12.2 How Are Aldehydes and Ketones Named? 12.9 How Are Aldehydes and Ketones Oxidized? 12.3 What Are the Physical Properties of Aldehydes 12.10 How Are Aldehydes and Ketones Reduced? and Ketones? 12.4 What Is the Most Common Reaction Theme of HOW TO Aldehydes and Ketones? 12.1 How to Predict the Product of a Grignard Reaction 12.5 What Are Grignard Reagents, and How Do They 12.2 How to Determine the Reactants Used to React with Aldehydes and Ketones? Synthesize a Hemiacetal or Acetal 12.6 What Are Hemiacetals and Acetals? 12.7 How Do Aldehydes and Ketones React with CHEMICAL CONNECTIONS Ammonia and Amines? 12A A Green Synthesis of Adipic Acid IN THIS AND several of the following chapters, we study the physical and chemical properties of compounds containing the carbonyl group, C O. Because this group is the functional group of aldehydes, ketones, and carboxylic acids and their derivatives, it is one of the most important functional groups in organic chemistry and in the chemistry of biological systems. The chemical properties of the carbonyl group are straightforward, and an understanding of its characteristic reaction themes leads very quickly to an understanding of a wide variety of organic reactions. -

REGISTRY Database Summary Sheet (DBSS)
SM REGISTRY/ZREGISTRY (CAS REGISTRY ) Subject • All types of inorganic and organic substances, including alloys, coordination Coverage compounds, minerals, mixtures, polymers, salts, high throughput screening (HTS) compounds as well as nucleic acid and protein sequences • Substances included in REGISTRY meet the following criteria: - Identified by CAS as coming from a reputable source, including but not limited to patents, journals, chemical catalogs, and selected substance collections on the web - Described in largely unambiguous terms - Characterized by physical methods or described in a patent document example or claim - Consistent with the laws of atomic covalent organization • Experimental and predicted property data and tags and spectra data File Type Numeric, Structure Features Alerts (SDIs) Biweekly In addition, SMARTracker, an automatic crossfile current-awareness search, (SDI XFILE) using a REGISTRY search profile in CA, HCA, ZCA, CAplus, HCAplus or ZCAplus may be run weekly or biweekly (weekly is the default). CAS Registry Keep & Share SLART Number® Identifiers ® Learning Database STN Easy Structures Record • CAS Registry Numbers • Experimental and predicted property Content • CA index names and commonly used data and tags chemical names and trade names • Spectra • Molecular formulas • List of databases and regulatory listings • Structure diagrams containing information on the substances • Sequence data • Super roles and document type SM • Alloy composition tables information from CAplus • Classes for polymers • Displayable information for 10 most recent references for substances • Ring analysis data File Size More than 140 million organic and inorganic substances; more than 71.1 million sequences (4/18) Coverage Early 1800s to the present Updates Daily Language English Database Chemical Abstracts Service Producer 2540 Olentangy River Road P.O. -

New Insights Into the Mechanism of Schiff Base Synthesis from Aromatic
A peer-reviewed version of this preprint was published in PeerJ on 15 December 2020. View the peer-reviewed version (peerj.com/articles/ochem-4), which is the preferred citable publication unless you specifically need to cite this preprint. Silva PJ. 2020. New insights into the mechanism of Schiff base synthesis from aromatic amines in the absence of acid catalyst or polar solvents. PeerJ Organic Chemistry 2:e4 https://doi.org/10.7717/peerj-ochem.4 New insights into the mechanism of Schiff base synthesis from aromatic amines in the absence of acid catalyst or polar solvents Pedro J Silva Corresp. 1 1 FP-ENAS/Fac. de Ciências da Saúde, Universidade Fernando Pessoa, Porto, Portugal Corresponding Author: Pedro J Silva Email address: [email protected] Extensive computational studies of the imine synthesis from amines and aldehydes in water have shown that the large-scale structure of water is needed to afford appropriate charge delocalisation and enable sufficient transition state stabilisation. These insights cannot, however, be applied to the understanding of the reaction pathway in apolar solvents due their inability to form extensive hidrogen-bonding networks. In this work, we perform the first computational studies of this reaction in apolar conditions. This density- functional study of the reaction of benzaldehyde with four closely related aromatic amines (aniline, o-toluidine, m-toluidine and p-toluidine) shows that an additional molecule of amine may provide enough stabilization of the first transition state even in the absence of a hydrogen bonding network. Our computations also show that the second reaction step cannot take place unless an extra proton is added to the system but, crucially, that reaction rate is so high that even picomolar amounts of protonated base are enough to achieve realistic rates. -

Exam 1 (February 23, 2004) ID# ______
Chemistry 211 Name ___________________________ Exam 1 (February 23, 2004) ID# ___________________________ 10 1. You desire to synthesize 3-ethyl-3-pentanol starting with an ester. (i) What would be the name of the ester, and what is the name for the Grignard reagent (e.g., methyl magnesium bromide)? (ii) For the carbons shown in the product, show plausible hydrocarbons that you could start with to produce the ester and the Grignard reagent (as in a retrosynthesis). 12 2. (i) Show the step-by-step process required to produce propyllithium, which requires a free radical reaction mechanism, . (ii) Show the complete reaction mechanism for reaction between propyllithium and the correct ketone to produce 3-propyl-3-pentanol. (iii) Propose a possible reaction mechanism by which dipropyl cuprate (Cu+ with two propyl groups attached) could react with ethyl bromide to produce a new hydrocarbon. (This is a thinking exercise! So, think! () 8 3. As mentioned in the text, diethyl ether, pentane, and 1-butanol have similar molar masses, but different physical properties. Boiling points are 35oC, 36oC, and 117oC, respectively. Their respective solubilities in water are 7.5g/100mL, insoluble, and 9g/100mL. (i) Draw structures for each of these compounds. (ii) Justify the observed boiling points and their solubilities. 16 4. Draw structures of the following compounds 2,3-heptanediol isopropyllithium benzylmagnesium bromide benzoic acid benzaldehylde dimethyl sulfide t-butyl methanoate dibutyl ketone 12 5. Alcohols can be oxidized to produce other compounds, and can be produced by reduction. For the reactions shown below, show the structure for the expected product (if reaction does not occur, state: No Reaction) when treated with the indicated oxidizing or reducing agents. -

Chapter 14 – Aldehydes and Ketones
Chapter 14 – Aldehydes and Ketones 14.1 Structures and Physical Properties of Aldehydes and Ketones Ketones and aldehydes are related in that they each possess a C=O (carbonyl) group. They differ in that the carbonyl carbon in ketones is bound to two carbon atoms (RCOR’), while that in aldehydes is bound to at least one hydrogen (H2CO and RCHO). Thus aldehydes always place the carbonyl group on a terminal (end) carbon, while the carbonyl group in ketones is always internal. Some common examples include (common name in parentheses): O O H HH methanal (formaldehyde) trans-3-phenyl-2-propenal (cinnamaldehyde) preservative oil of cinnamon O O propanone (acetone) 3-methylcyclopentadecanone (muscone) nail polish remover a component of one type of musk oil Simple aldehydes (e.g. formaldehyde) typically have an unpleasant, irritating odor. Aldehydes adjacent to a string of double bonds (e.g. 3-phenyl-2-propenal) frequently have pleasant odors. Other examples include the primary flavoring agents in oil of bitter almond (Ph- CHO) and vanilla (C6H3(OH)(OCH3)(CHO)). As your book says, simple ketones have distinctive odors (similar to acetone) that are typically not unpleasant in low doses. Like aldehydes, placing a collection of double bonds adjacent to a ketone carbonyl generally makes the substance more fragrant. The primary flavoring agent in oil of caraway is just a such a ketone. 2 O oil of carraway Because the C=O group is polar, small aldehydes and ketones enjoy significant water solubility. They are also quite soluble in typical organic solvents. 14.2 Naming Aldehydes and Ketones Aldehydes The IUPAC names for aldehydes are obtained by using rules similar to those we’ve seen for other functional groups (e.g. -

Carbonyl Compounds
CARBONYL COMPOUNDS PART-4, PPT-4, SEM-3 Dr. Kalyan Kumar Mandal Associate Professor St. Paul’s C. M. College Kolkata CONTENTS: CARBONYL COMPOUNDS PART-4 • Formation of Acetal/Ketal • Formation of Thioacetal Reaction of Carbonyl Compounds with Alcohols • Carbonyl compounds react with alcohols. The product of this reaction is known as a hemiacetal, because it is halfway to an acetal. This reaction is analogous to hydrate formation from aldehydes and ketones. The mechanism follows in the footsteps of hydrate formation: ROH is used instead of HOH (water). This Lecture is prepared by Dr. K. K. Mandal, SPCMC, Kolkata Formation of Cyclic Hemiacetal • Hemiacetal formation is reversible, and they are stabilized by the same special structural features as those of hydrates. However, hemiacetals can also gain stability by being cyclic. • Cyclic hemiacetal is formed when the carbonyl group and the attacking hydroxyl group are part of the same molecule. The reaction is an intramolecular (within the same molecule) addition, as opposed to the intermolecular (between two molecules) ones. This is an example of ring-chain tautomerism. This Lecture is prepared by Dr. K. K. Mandal, SPCMC, Kolkata Formation of Cyclic Hemiacetal • Although the cyclic hemiacetal is more stable, it is still in equilibrium with some of the open-chain hydroxyaldehyde form. Its stability, and how easily it forms, depend on the size of the ring. • Five- and six-membered rings involve less strain (their bonds are free to adopt 109° or 120° angles) in comparison to the three- membered rings, and therefore five or six-membered hemiacetals are very common. -

Synthesis of Aniline and Benzaldehyde Derivatives From
Electronic Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2011 Supporting Information Fe2O3-supported nano-gold catalyzed one-pot synthesis of N-alkylated anilines from nitroarenes and alcohols * Qiling Peng, Yan Zhang, Feng Shi , Youquan Deng Centre for Green Chemistry and Catalysis, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou, 730000, China *corresponding author: Tel.: +86-931-4968142; Fax: +86-931-4968116; E-mail: [email protected] 1. Materials and Methods 2. Chlorine concentration measurement 3. Catalyst preparation 4. Table S2. ICP-AES and BET data of various catalysts 5. General procedure for the reaction of nitrobenzene with d7-benzyl alcohol 6. General procedure for the H-D exchange reaction between aniline and D2O 7. Fig. S2. XRD patterns of the catalysts in the experiments. 8. XPS spectra of catalyst samples 9. HRTEM images of the catalyst samples 10.GC-MS spectra of compounds observed by GC-MS 11. Compounds characterization and NMR spectra Electronic Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2011 1. Materials and Methods All solvent and chemicals were obtained commercially and were used as received. NMR spectra were measured using a Bruker ARX 400 or ARX 100 spectrometer at 400 MHz (1H) and 100 MHz 13 ( C). All spectra were recorded in CDCl3 and chemical shifts (δ) are reported in ppm relative to tetramethylsilane referenced to the residual solvent peaks. Mass spectra were in general recorded on an HP 6890/5973 GC-MS. High-resolution TEM analysis was carried out on a JEM 2010 operating at 200 KeV. -

Grignard Reaction: Synthesis of Triphenylmethanol
*NOTE: Grignard reactions are very moisture sensitive, so all the glassware in the reaction (excluding the work-up) should be dried in an oven with a temperature of > 100oC overnight. The following items require oven drying. They should be placed in a 150mL beaker, all labeled with a permanent marker. 1. 5mL conical vial (AKA: Distillation receiver). 2. Magnetic spin vane. 3. Claisen head. 4. Three Pasteur pipettes. 5. Two 1-dram vials (Caps EXCLUDED). 6. One 2-dram vial (Caps EXCLUDED). 7. Glass stirring rod 8. Adaptor (19/22.14/20) Grignard Reaction: Synthesis of Triphenylmethanol Pre-Lab: In the “equations” section, besides the main equations, also: 1) draw the equation for the production of the byproduct, Biphenyl. 2) what other byproduct might occur in the reaction? Why? In the “observation” section, draw data tables in the corresponding places, each with 2 columns -- one for “prediction” (by answering the following questions) and one for actual drops or observation. 1) How many drops of bromobenzene should you add? 2) How many drops of ether will you add to flask 2? 3) 100 µL is approximately how many drops? 4) What are the four signs of a chemical reaction? (Think back to Chem. 110) 5) How do the signs of a chemical reaction apply to this lab? The Grignard reaction is a useful synthetic procedure for forming new carbon- carbon bonds. This organometallic chemical reaction involves alkyl- or aryl-magnesium halides, known as Grignard 1 reagents. Grignard reagents are formed via the action of an alkyl or aryl halide on magnesium metal. -

Organic Chemistry Laboratory II Preparation of Triphenylmethanol (Grignard Reaction) Experiment Procedure (Printable Pdf Format)
grigdes http://www.organicchem.org/oc2web/lab/exp/grig/grigdesbenzophenone... Organic Chemistry II | Lecture | Laboratory Organic Chemistry Laboratory II Preparation of Triphenylmethanol (Grignard Reaction) Experiment Procedure (Printable pdf format) Introduction In this two-week experiment, triphenylmethanol will be synthesized through a Grignard reaction. Students will work individually to prepare the Grignard reagent by reacting bromobenzene with solid magnesium in diethylether. The prepared Grignard reagent, phenylmagnesium bromide, will then be combined with bezophenone to form the desired triphenylmethanol product. Procedure NOTE: All glassware must be extremely dry! Place glassware in the drying oven for at least 10 minutes before beginning the reaction. Preparation of the Grignard Reagent Add ~0.5g of magnesium turnings and a magnetic stir bar to a dry 100 ml round-bottomed flask, and place the flask in the drying oven for 30 minutes. Remove the flask from the oven, clamp it to a ring stand, insert a reflux condenser, fit the flask into a heating mantle and immediately attach a drying tube to the top of the reflux condenser. Position the flask in the heating mantle on a stirrer/hotplate. Set the reaction up on the side of the hood near to the faucet labeled for distillation. Allow the reaction flask to cool to room temperature completely before proceeding. While the flask is cooling, place a 150ml beaker into the drying oven. While the flask is cooling, add 3.5 g of dry bromobenzene to a dry 50 mL Erlenmeyer flask and dissolve it in 5.0 mL of anhydrous diethyl ether. Use a glass powder funnel to dispense the bromobenze and ether to the Erlenmyer flask. -

Reactions of Aromatic Compounds Just Like an Alkene, Benzene Has Clouds of Electrons Above and Below Its Sigma Bond Framework
Reactions of Aromatic Compounds Just like an alkene, benzene has clouds of electrons above and below its sigma bond framework. Although the electrons are in a stable aromatic system, they are still available for reaction with strong electrophiles. This generates a carbocation which is resonance stabilized (but not aromatic). This cation is called a sigma complex because the electrophile is joined to the benzene ring through a new sigma bond. The sigma complex (also called an arenium ion) is not aromatic since it contains an sp3 carbon (which disrupts the required loop of p orbitals). Ch17 Reactions of Aromatic Compounds (landscape).docx Page1 The loss of aromaticity required to form the sigma complex explains the highly endothermic nature of the first step. (That is why we require strong electrophiles for reaction). The sigma complex wishes to regain its aromaticity, and it may do so by either a reversal of the first step (i.e. regenerate the starting material) or by loss of the proton on the sp3 carbon (leading to a substitution product). When a reaction proceeds this way, it is electrophilic aromatic substitution. There are a wide variety of electrophiles that can be introduced into a benzene ring in this way, and so electrophilic aromatic substitution is a very important method for the synthesis of substituted aromatic compounds. Ch17 Reactions of Aromatic Compounds (landscape).docx Page2 Bromination of Benzene Bromination follows the same general mechanism for the electrophilic aromatic substitution (EAS). Bromine itself is not electrophilic enough to react with benzene. But the addition of a strong Lewis acid (electron pair acceptor), such as FeBr3, catalyses the reaction, and leads to the substitution product. -
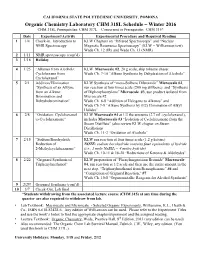
318 Lab Schedule
CALIFORNIA STATE POLYTECHNIC UNIVERSITY, POMONA Organic Chemistry Laboratory CHM 318L Schedule – Winter 2016 CHM 318L Prerequisites: CHM 317L Concurrent or Prerequisite: CHM 315* Date Experiment/Activity Experimental Procedure and Required Reading 1 1/4 Check-in. Introduction to KLW Chapters on “Infrared Spectroscopy” and “Nuclear NMR Spectroscopy Magnetic Resonance Spectroscopy” (KLW = Williamson text) Wade Ch. 12 (IR) and Wade Ch. 13 (NMR) 2 1/11 NMR spectroscopy (cont’d) 3 1/18 Holiday 4 1/25 “Alkenes from Alcohols: KLW Macroscale #2, 20 g scale, skip toluene chaser Cyclohexene from Wade Ch. 7-10 “Alkene Synthesis by Dehydration of Alcohols” Cyclohexanol” 5 2/1 Addition/Elimination KLW Synthesis of “meso-Stilbene Dibromide” Microscale #2, “Synthesis of an Alkyne run reaction at four times scale (200 mg stillbene); and “Synthesis from an Alkene: of Diphenylacetylene” Microscale #3, use product isolated from Bromination and Microscale #2 Dehydrobromination” Wade Ch. 8-8 “Addition of Halogens to Alkenes” and Wade Ch 7-9 “Alkene Synthesis by (E2) Elimination of Alkyl Halides” 6 2/8 “Oxidation: Cyclohexanol KLW Macroscale #4 at 1/3 the amounts (2.7 mL cyclohexanol), to Cyclohexanone” includes Macroscale #3 “Isolation of Cyclohexanone from the Steam Distillate” (also review KLW chapter on Steam Distillation) Wade Ch. 11-2 “Oxidation of Alcohols” 7 2/15 “Sodium Borohydride KLW run reaction at four times scale (1.2 g ketone) Reduction of NOTE: sodium borohydride contains four equivalents of hydride 2-Methylcyclohexanone” (i.e., 1 mole NaBH4 = 4 moles hydride) Wade Ch. 10-11 & 18-20 “Reductions of Ketones & Aldehydes” 8 2/22 “Grignard Synthesis of KLW preparation of “Phenylmagnesium Bromide” Macroscale Triphenylmethanol” #4, run reaction at 1/2 scale and then use the entire amount in the next step, “Triphenylmethanol from Benzophenone” #6 and “Completion of Grignard Reaction,” #7 Wade Ch.