1.1 10 Oxidation of Alcohols and Aldehydes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Photocatalytic Performance of Cuxo/Tio2 Deposited by Hipims on Polyester Under Visible Light Leds: Oxidants, Ions Effect, and Reactive Oxygen Species Investigation
materials Article Photocatalytic Performance of CuxO/TiO2 Deposited by HiPIMS on Polyester under Visible Light LEDs: Oxidants, Ions Effect, and Reactive Oxygen Species Investigation Hichem Zeghioud 1 , Aymen Amine Assadi 2,*, Nabila Khellaf 3, Hayet Djelal 4, Abdeltif Amrane 2 and Sami Rtimi 5,* 1 Department of Process Engineering, Faculty of Engineering, Badji Mokhtar University, P.O. Box 12, 23000 Annaba, Algeria; [email protected] 2 Ecole Nationale Supérieure de Chimie de Rennes, Université de Rennes 1, CNRS, UMR 6226, Allée de Beaulieu, CS 50837, 35708 Rennes CEDEX 7, France; [email protected] 3 Laboratory of Organic Synthesis-Modeling and Optimization of Chemical Processes, Badji Mokhtar University, P.O. Box 12, 23000 Annaba, Algeria; [email protected] 4 Ecole des Métiers de l’Environnement, Campus de Ker Lann, 35170 Bruz, France; [email protected] 5 Ecole Polytechnique Fédérale de Lausanne, EPFL-STI-LTP, Station 12, CH-1015 Lausanne, Switzerland * Correspondence: [email protected] (A.A.A.); sami.rtimi@epfl.ch (S.R.) Received: 29 December 2018; Accepted: 22 January 2019; Published: 29 January 2019 Abstract: In the present study, we propose a new photocatalytic interface prepared by high-power impulse magnetron sputtering (HiPIMS), and investigated for the degradation of Reactive Green 12 (RG12) as target contaminant under visible light light-emitting diodes (LEDs) illumination. The CuxO/TiO2 nanoparticulate photocatalyst was sequentially sputtered on polyester (PES). The photocatalyst formulation was optimized by investigating the effect of different parameters such as the sputtering time of CuxO, the applied current, and the deposition mode (direct current magnetron sputtering, DCMS or HiPIMS). -

Working with Hazardous Chemicals
A Publication of Reliable Methods for the Preparation of Organic Compounds Working with Hazardous Chemicals The procedures in Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices. In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red “Caution Notes” within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices. The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein. -

Safety Data Sheet
SAFETY DATA SHEET Preparation Date: 06/23/2015 Revision Date: 10/27/2015 Revision Number: G2 1. IDENTIFICATION Product identifier Product code: P1282 Product Name: POTASSIUM DICHROMATE, CRYSTAL, REAGENT, ACS Other means of identification Synonyms: Bichromate of potash Chromium potassium oxide (K2Cr2O7) Dichromic acid dipotassium salt Dipotassium bichromate Dipotassium dichromate Dipotassium dichromium heptaoxide Iopezite Kaliumdichromat [German] Potassium bichromate Potassium chromate (K2Cr2O7) Potassium dichromate Potassium dichromate (K2(Cr2O7)) Potassium dichromate (K2Cr2O7) Potassium dichromate(VI) SRM 935a CAS #: 7778-50-9 RTECS # HX7680000 CI#: Not available Recommended use of the chemical and restrictions on use Recommended use: Analytical reagent. In tanning, printing, painting, electroplating and pyrotechnics. Uses advised against No information available Supplier: Spectrum Chemical Mfg. Corp 14422 South San Pedro St. Gardena, CA 90248 (310) 516-8000 Order Online At: https://www.spectrumchemical.com Emergency telephone number Chemtrec 1-800-424-9300 Contact Person: Martin LaBenz (West Coast) Contact Person: Ibad Tirmiz (East Coast) 2. HAZARDS IDENTIFICATION Classification This chemical is considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Product code: P1282 Product name: POTASSIUM 1 / 16 DICHROMATE, CRYSTAL, REAGENT, ACS Acute toxicity - Oral Category 2 Acute toxicity - Dermal Category 1 Acute toxicity - Inhalation (Gases) Category 2 Acute toxicity - Inhalation (Dusts/Mists) Category 2 Skin -
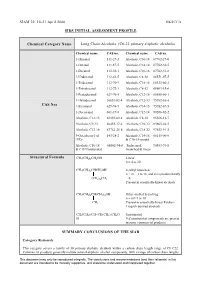
Long Chain Alcohols (C6-22 Primary Aliphatic Alcohols)
SIAM 22, 18-21 April 2006 UK/ICCA SIDS INITIAL ASSESSMENT PROFILE Chemical Category Name Long Chain Alcohols (C6-22 primary aliphatic alcohols) Chemical name CAS no. Chemical name CAS no. 1-Hexanol 111-27-3 Alcohols, C16-18 67762-27-0 1-Octanol 111-87-5 Alcohols, C14-18 67762-30-5 1-Decanol 112-30-1 Alcohols, C10-16 67762-41-8 1-Undecanol 112-42-5 Alcohols, C8-18 68551-07-5 1-Tridecanol 112-70-9 Alcohols, C14-16 68333-80-2 1-Tetradecanol 112-72-1 Alcohols, C6-12 68603-15-6 1-Pentadecanol 629-76-5 Alcohols, C12-16 68855-56-1 1-Hexadecanol 36653-82-4 Alcohols, C12-13 75782-86-4 CAS Nos 1-Eicosanol 629-96-9 Alcohols, C14-15 75782-87-5 1-Docosanol 661-19-8 Alcohols, C12-14 80206-82-2 Alcohols, C12-15 63393-82-8 Alcohols, C8-10 85566-12-7 Alcohols, C9-11 66455-17-2 Alcohols, C10-12 85665-26-5 Alcohols, C12-18 67762-25-8 Alcohols, C18-22 97552-91-5 9-Octadecen-1-ol 143-28-2 Alcohols, C14-18. 68155-00-0 (9Z)- & C16-18-unsatd Alcohols, C16-18 68002-94-8 Tridecanol, 90583-91-8 & C18 Unsaturated branched & linear Structural Formula CH3(CH2)nCH2OH Linear n = 4 to 20 CH3(CH2)nCHCH2OH 2-Alkyl branched n + m = 3 to 18, and m is predominantly (CH2)mCH3 = 0. Present in essentially-linear alcohols CH3(CH2)nCH(CH2)mOH Other-methyl branching n + m= 9 or 10 CH3 Present in essentially-linear Fischer- Tropsch derived alcohols CH3(CH2)7CH=CH(CH2)7CH2O Unsaturated H 9-Z unsaturated components are present in some commercial products. -

Transcription 12.01.12
Lecture 2B • 01/12/12 We covered three different reactions for converting alcohols into leaving groups. One was to turn an alcohol into an alkyl chloride, that was using thionyl chloride. Second reaction was using tosyl chloride; the primary difference between those two reactions is one of stereochemistry. An inversion of stereochemistry does occur if you use thionyl chloride, because it does affect the carbon-oxygen bond, but because forming a tosylate does not touch the carbon-oxygen bond, only the oxygen- hydrogen bond, there’s no change in stereochemistry there. We then saw phosphorus tribromide that reacts very similarly to the thionyl chloride; you get an alkyl bromide instead, but it also has inversion of configuration. The last reaction is not a new mechanism, it is just an Sn2 reaction, it’s called the Finkelstein reaction. Really this works, in a sense, off of Le Châtelier’s principle. In solution, in theory, sodium iodide can displace bromide, but sodium bromide can displace iodide, so you can have an Sn2 reaction that goes back and forth and back and forth and back and forth. Except, sodium iodide is somewhat soluble in acetone, while sodium chloride and sodium bromide are not. So, in fact, one of the things that we get out of this as a by-product is sodium bromide, which, again, is not soluble in acetone and therefore precipitates out and is no longer part of the reaction mixture, so there’s no reverse reaction possible. Because of this solubility trick, it allows this reaction to be pulled forward, which means you can get the alkyl iodide. -

Manufacturing of Potassium Permanganate Kmno4 This Is the Most Important and Well Known Salt of Permanganic Acid
Manufacturing of Potassium Permanganate KMnO4 This is the most important and well known salt of permanganic acid. It is prepared from the pyrolusite ore. It is prepared by fusing pyrolusite ore either with KOH or K2CO3 in presence of atmospheric oxygen or any other oxidising agent such as KNO3. The mass turns green with the formation of potassium manganate, K2MnO4. 2MnO2 + 4KOH + O2 →2K2MnO4 + 2H2O 2MnO2 + 2K2CO3 + O2 →2K2MnO4 + 2CO2 The fused mass is extracted with water. The solution is now treated with a current of chlorine or ozone or carbon dioxide to convert manganate into permanganate. 2K2MnO4 + Cl2 → 2KMnO4 + 2KCl 2K2MnO4 + H2O + O3 → 2KMnO4 + 2KOH + O2 3K2MnO4 + 2CO2 → 2KMnO4 + MnO2 + 2K2CO3 Now-a-days, the conversion is done electrolytically. It is electrolysed between iron cathode and nickel anode. Dilute alkali solution is taken in the cathodic compartment and potassium manganate solution is taken in the anodic compartment. Both the compartments are separated by a diaphragm. On passing current, the oxygen evolved at anode oxidises manganate into permanganate. At anode: 2K2MnO4 + H2O + O → 2KMnO4 + 2KOH 2- - - MnO4 → MnO4 + e + - At cathode: 2H + 2e → H2 Properties: It is purple coloured crystalline compound. It is fairly soluble in water. When heated alone or with an alkali, it decomposes evolving oxygen. 2KMnO4 → K2MnO4 + MnO2 + O2 4KMnO4 + 4KOH → 4K2MnO4 + 2H2O + O2 On treatment with conc. H2SO4, it forms manganese heptoxide via permanganyl sulphate which decomposes explosively on heating. 2KMnO4+3H2SO4 → 2KHSO4 + (MnO3)2SO4 + 2H2O (MnO3)2SO4 + H2O → Mn2O7 + H2SO4 Mn2O7 → 2MnO2 + 3/2O2 Potassium permanganate is a powerful oxidising agent. A mixture of sulphur, charcoal and KMnO4 forms an explosive powder. -

Chemical Compatibility Guide
Chemical Compatibility Guide Guide Applicable to the Following: PIG Portable Spill Containment Pool Guide Information This report is offered as a guide and was developed from information which, to the best of New Pig’s knowledge, was reliable and accurate. Due to variables and conditions of application beyond New Pig’s control, none of the data shown in this guide is to be construed as a guarantee, expressed or implied. New Pig assumes no responsibility, obligation, or liability in conjunction with the use or misuse of the information. PIG Spill Containment Pools are constructed from PVC-coated polyester fabric. The chemical resistance guide that follows shows the chemical resistance for the PVC layer only. This guide has been compiled to provide the user with general chemical resistance information. It does not reflect actual product testing. Ratings / Key or Ratings – Chemical Effect 1. Satisfactory to 72°F (22°C) 2. Satisfactory to 120°F (48°C) A = Excellent D = Severe Effect, not recommended for ANY use. B = Good — Minor Effect, slight corrosion or discoloration. N/A = Information not available. C = Fair — Moderate Effect, not recommended for continuous use. Softening, loss of strength, swelling may occur. Due to variables and conditions beyond our control, New Pig cannot guarantee that this product(s) will work to your satisfaction. To ensure effectiveness and your safety, we recommend that you conduct compatibility and absorption testing of your chemicals with this product prior to purchase. For additional questions or information, -

Perkin's Mauve: the History of the Chemistry
REFLECTIONS Perkin’s Mauve: The History of the Chemistry Andrew Filarowski Those of us who owe our living in part to the global dyestuff and chemical industry should pause today and remember the beginnings of this giant industry which started 150 years ago today with William Perkins’ discovery of mauveine whilst working in his home laboratory during the Easter holiday on April 28, 1856. Prior to this discovery, all textiles were dyed with natural dyestuffs and pigments. What did Perkin’s Reaction Entail? William Henry Perkin carried out his experiments at his home laboratory in the Easter break of 1856. He was trying to produce quinine (C20H24N2O2). This formula was known but not the structural formula. Because chemistry was in such an early stage of development Perkin thought that by simply balancing the masses (simple additive and subtractive chemistry) in an equation he would obtain the required compound. He therefore believed that if he took two allyltoluidine molecules, C10H13N, and oxidised them with three oxygen atoms (using potassium dichromate) he would get quinine (C20H24N2O2) and water. 2 (C10H13N) + 3O C20H24N2O2 + H2O It is unsurprising to us now but Perkin reported “that no quinine was formed, but only a dirty reddish brown precipitate.” However, he continued in his trials and decided to use aniline (C6H5NH2) and its sulphate, and to oxidise them using potassium dichromate. This produced a black precipitate that Perkin at first took to be a failed experiment, but he noticed on cleaning his equipment with alcohol that a coloured solution was obtained. Perkin’s Patent W H Perkin filed his patent on the 26th August 1856 for “Producing a new colouring matter for the dyeing with a lilac or purple color stuffs of silk, cotton, wool, or other materials.” (sic) Patent No. -

Reactions of Alcohols & Ethers 1
REACTIONS OF ALCOHOLS & ETHERS 1. Combustion (Extreme Oxidation) alcohol + oxygen carbon dioxide + water 2 CH3CH2OH + 6 O2 4 CO2 + 6 H2O 2. Elimination (Dehydration) ° alcohol H 2 S O 4/ 1 0 0 C alkene + water H2SO4/100 ° C CH3CH2CH2OH CH3CH=CH2 + H2O 3. Condensation ° excess alcohol H 2 S O 4 / 1 4 0 C ether + water H2SO4/140 ° C 2 CH3CH2OH CH3CH2OCH2CH3 + H2O 4. Substitution Lucas Reagent alcohol + hydrogen halide Z n C l2 alkyl halide + water ZnCl2 CH3CH2OH + HCl CH3CH2Cl + H2O • This reaction with the Lucas Reagent (ZnCl2) is a qualitative test for the different types of alcohols because the rate of the reaction differs greatly for a primary, secondary and tertiary alcohol. • The difference in rates is due to the solubility of the resulting alkyl halides • Tertiary Alcohol→ turns cloudy immediately (the alkyl halide is not soluble in water and precipitates out) • Secondary Alcohol → turns cloudy after 5 minutes • Primary Alcohol → takes much longer than 5 minutes to turn cloudy 5. Oxidation • Uses an oxidizing agent such as potassium permanganate (KMnO4) or potassium dichromate (K2Cr2O7). • This reaction can also be used as a qualitative test for the different types of alcohols because there is a distinct colour change. dichromate → chromium 3+ (orange) → (green) permanganate → manganese (IV) oxide (purple) → (brown) Tertiary Alcohol not oxidized under normal conditions CH3 KMnO4 H3C C OH NO REACTION K2Cr2O7 CH3 tertbutyl alcohol Secondary Alcohol ketone + hydrogen ions H O KMnO4 H3C C CH3 + K2Cr2O7 C + 2 H H3C CH3 OH propanone 2-propanol Primary Alcohol aldehyde + water carboxylic acid + hydrogen ions O KMnO4 O H H KMnO H H 4 + CH3CH2CH2OH C C C H C C C H + 2 H K2Cr2O7 + H2O K Cr O H H H 2 2 7 HO H H 1-propanol propanal propanoic acid 6. -

Potassium Dichromate Safety Data Sheet According to Federal Register / Vol
Potassium Dichromate Safety Data Sheet according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations Date of issue: 12/11/2014 Version: 1.0 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product form : Substance Substance name : Potassium Dichromate Chemical name : potassium dichromate CAS No : 7778-50-9 Product code : LC18940 Formula : K2Cr2O7 1.2. Relevant identified uses of the substance or mixture and uses advised against Use of the substance/mixture : For laboratory and manufacturing use only. 1.3. Details of the supplier of the safety data sheet LabChem Inc Jackson's Pointe Commerce Park Building 1000, 1010 Jackson's Pointe Court Zelienople, PA 16063 - USA T 412-826-5230 - F 724-473-0647 [email protected] - www.labchem.com 1.4. Emergency telephone number Emergency number : CHEMTREC: 1-800-424-9300 or 011-703-527-3887 SECTION 2: Hazards identification 2.1. Classification of the substance or mixture Classification (GHS-US) Ox. Sol. 2 H272 Acute Tox. 3 (Oral) H301 Acute Tox. 4 (Dermal) H312 Acute Tox. 2 (Inhalation) H330 Skin Corr. 1B H314 Resp. Sens. 1 H334 Skin Sens. 1 H317 Muta. 1B H340 Carc. 1B H350 Repr. 1B H360 STOT RE 1 H372 Aquatic Acute 1 H400 Aquatic Chronic 1 H410 Full text of H-phrases: see section 16 2.2. Label elements GHS-US labeling Hazard pictograms (GHS-US) : GHS03 GHS05 GHS06 GHS08 GHS09 Signal word (GHS-US) : Danger Hazard statements (GHS-US) : H272 - May intensify fire; oxidizer H301 - Toxic if swallowed H312 - Harmful in contact with skin H314 - Causes severe skin burns and eye damage H317 - May cause an allergic skin reaction H330 - Fatal if inhaled H334 - May cause allergy or asthma symptoms or breathing difficulties if inhaled H340 - May cause genetic defects H350 - May cause cancer H360 - May damage fertility or the unborn child H372 - Causes damage to organs (kidneys, liver, Skin) through prolonged or repeated 12/11/2014 EN (English US) Page 1 Potassium Dichromate Safety Data Sheet according to Federal Register / Vol. -

Chapter 18 Ethers and Epoxides; Thiols and Sulfides Ethers
Chapter 18 Ethers and Epoxides; Thiols and Sulfides Ethers • Ethers (R–O–R’): – Organic derivatives of water, having two organic groups bonded to the same oxygen atom © 2016 Cengage Learning 2 NAMES AND PROPERTIES OF ETHERS 3 Nomenclature: Common Names • Simple ethers are named by identifying two organic substituents and adding the word ether – Name the groups in alphabetical order – Symmetrical: Use dialkyl or just alkyl © 2016 Cengage Learning 4 Nomenclature: IUPAC Names • The more complex alkyl group is the parent name • The group with the oxygen becomes an alkoxy group © 2016 Cengage Learning 5 Nomenclature: Cyclic Ethers (Heterocycles) • Heterocyclic: Oxygen is part of the ring. O • Epoxides (oxiranes) H2C CH2 O • Oxetanes • Furans (Oxolanes) O O • Pyrans (Oxanes) O O O • Dioxanes O © 2013 Pearson Education, Inc. 6 Epoxide Nomenclature • Name the starting alkene and add “oxide” © 2013 Pearson Education, Inc. 7 Epoxide Nomenclature • The oxygen can be treated as a substituent (epoxy) on the compound • Use numbers to specify position • Oxygen is 1, the carbons are 2 and 3 • Substituents are named in alphabetical order © 2013 Pearson Education, Inc. 8 Properties of Ethers • Possess nearly the same geometry as water – Oxygen atom is sp3-hybridized – Bond angles of R–O–R bonds are approximately tetrahedral • Polar C—O bonds © 2013 Pearson Education, Inc. 9 Properties of Ethers: Hydrogen Bond • Hydrogen bond is a attractive interaction between an electronegative atom and a hydrogen atom bonded to another electronegative atom • Ethers cannot hydrogen bond with other ether molecules, so they have a lower boiling point than alcohols • Ether molecules can hydrogen bond with water and alcohol molecules • They are hydrogen bond acceptors © 2013 Pearson Education, Inc. -

Soln-2212-PT3-S20-Ch-16-18.Pdf
1. 2. H2SO4 H2SO4 H2SO41) BH3, THF 1) BH3, THF 1) BH3, THF - - - 2) OH , H2O2, H2O2) OH , H2O2, H2O 2) OH , H2O2, H2O OH OH OH 3. OH OH OH H2SO4 1) BH3, THF - 2) OH , H2O2, H2O OH PBr3 PBr3 PBr3 Pyridine Pyridine Pyridine OH PBr3 Pyridine O O O Mg Mg Mg HO HO HO1) 1) 1) Ether Ether Ether 2) HCl 2) HCl 2) HCl Br Br Br MgBr MgBr MgBr O Mg HO 1) Ether 2) HCl Br 4. MgBr **This reaction reduces an aldehyde to a primary alcohol and a ketone to a secondary alcohol. Since only one EQ of reagent is given the most reactive functional group will be reduced (in this case the aldehyde) **ketones are less reactive than aldehydes and the more steric hindrance surrounding the ketone the less reactive it is. Note: NaBH4 reduces ketones but is too weak to reduce an ester/double/triple bonds 5. ** With these reagents a tertiary amide will be reduced to a tertiary amine, a secondary amide will be reduced to a secondary amine, and a primary amide will be reduced to a primary amine 6. ** Recall from chapter 7, OChem 1 H2 and Lindlars catalyst always reduces an alkyne to a CIS ALKENE ** Li/Na and liquid NH3 always reduces an alkyne to a TRANS ALKENE 7. **A ketone/aldehyde reacts with primary amines in trace acid to produce an imine, and reacts with a secondary amine in trace acid to produce an enamine (double bond forms between the alpha and beta carbons) **Sodium cyanoborohydride (NaBH3CN) is used to reduce imines/enamines back to their respective secondary/tertiary amines 8.