Reactions of Alcohols & Ethers 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

EH&S COVID-19 Chemical Disinfectant Safety Information
COVID-19 CHEMICAL DISINFECTANT SAFETY INFORMATION Updated June 24, 2020 The COVID-19 pandemic has caused an increase in the number of disinfection products used throughout UW departments. This document provides general information about EPA-registered disinfectants, such as potential health hazards and personal protective equipment recommendations, for the commonly used disinfectants at the UW. Chemical Disinfectant Base / Category Products Potential Hazards Controls ● Ethyl alcohol Highly flammable and could form explosive Disposable nitrile gloves Alcohols ● ● vapor/air mixtures. ● Use in well-ventilated areas away from o Clorox 4 in One Disinfecting Spray Ready-to-Use ● May react violently with strong oxidants. ignition sources ● Alcohols may de-fat the skin and cause ● Wear long sleeve shirt and pants ● Isopropyl alcohol dermatitis. ● Closed toe shoes o Isopropyl Alcohol Antiseptic ● Inhalation of concentrated alcohol vapor 75% Topical Solution, MM may cause irritation of the respiratory tract (Ready to Use) and effects on the central nervous system. o Opti-Cide Surface Wipes o Powell PII Disinfectant Wipes o Super Sani Cloth Germicidal Wipe 201 Hall Health Center, Box 354400, Seattle, WA 98195-4400 206.543.7262 ᅵ fax 206.543.3351ᅵ www.ehs.washington.edu ● Formaldehyde Formaldehyde in gas form is extremely Disposable nitrile gloves for Aldehydes ● ● flammable. It forms explosive mixtures with concentrations 10% or less ● Paraformaldehyde air. ● Medium or heavyweight nitrile, neoprene, ● Glutaraldehyde ● It should only be used in well-ventilated natural rubber, or PVC gloves for ● Ortho-phthalaldehyde (OPA) areas. concentrated solutions ● The chemicals are irritating, toxic to humans ● Protective clothing to minimize skin upon contact or inhalation of high contact concentrations. -

Photocatalytic Performance of Cuxo/Tio2 Deposited by Hipims on Polyester Under Visible Light Leds: Oxidants, Ions Effect, and Reactive Oxygen Species Investigation
materials Article Photocatalytic Performance of CuxO/TiO2 Deposited by HiPIMS on Polyester under Visible Light LEDs: Oxidants, Ions Effect, and Reactive Oxygen Species Investigation Hichem Zeghioud 1 , Aymen Amine Assadi 2,*, Nabila Khellaf 3, Hayet Djelal 4, Abdeltif Amrane 2 and Sami Rtimi 5,* 1 Department of Process Engineering, Faculty of Engineering, Badji Mokhtar University, P.O. Box 12, 23000 Annaba, Algeria; [email protected] 2 Ecole Nationale Supérieure de Chimie de Rennes, Université de Rennes 1, CNRS, UMR 6226, Allée de Beaulieu, CS 50837, 35708 Rennes CEDEX 7, France; [email protected] 3 Laboratory of Organic Synthesis-Modeling and Optimization of Chemical Processes, Badji Mokhtar University, P.O. Box 12, 23000 Annaba, Algeria; [email protected] 4 Ecole des Métiers de l’Environnement, Campus de Ker Lann, 35170 Bruz, France; [email protected] 5 Ecole Polytechnique Fédérale de Lausanne, EPFL-STI-LTP, Station 12, CH-1015 Lausanne, Switzerland * Correspondence: [email protected] (A.A.A.); sami.rtimi@epfl.ch (S.R.) Received: 29 December 2018; Accepted: 22 January 2019; Published: 29 January 2019 Abstract: In the present study, we propose a new photocatalytic interface prepared by high-power impulse magnetron sputtering (HiPIMS), and investigated for the degradation of Reactive Green 12 (RG12) as target contaminant under visible light light-emitting diodes (LEDs) illumination. The CuxO/TiO2 nanoparticulate photocatalyst was sequentially sputtered on polyester (PES). The photocatalyst formulation was optimized by investigating the effect of different parameters such as the sputtering time of CuxO, the applied current, and the deposition mode (direct current magnetron sputtering, DCMS or HiPIMS). -

Working with Hazardous Chemicals
A Publication of Reliable Methods for the Preparation of Organic Compounds Working with Hazardous Chemicals The procedures in Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices. In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red “Caution Notes” within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices. The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein. -

1.1 10 Oxidation of Alcohols and Aldehydes
1.1 10 Oxidation of alcohols and aldehydes By the end of this spread, you should be able to … 1Describe the oxidation of primary alcohols to form aldehydes and carboxylic acids. 1Describe the oxidation of secondary alcohols to form ketones. 1Describe the oxidation of aldehydes to form carboxylic acids. Key definition Oxidation of alcohols You will recall from your AS chemistry studies that primary and secondary alcohols can A redox reaction is one in which both reduction and oxidation take place. be oxidised using an oxidising agent. s !SUITABLEOXIDISINGAGENTISASOLUTIONCONTAININGACIDIlEDDICHROMATEIONS + 2− H /Cr2O7 . s 4HEOXIDISINGMIXTURECANBEMADEFROMPOTASSIUMDICHROMATE +2Cr2O7, and sulfuric acid, H2SO. During the reaction, the acidified potassium dichromate changes from orange to green. Remember that tertiary alcohols are not oxidised by acidified dichromate ions. Primary alcohols Primary alcohols can be oxidised to aldehydes and to carboxylic acids (see AS Chemistry spread 2.2.3). H H H O H O Oxidation Oxidation H CCOH H CC H CC H H H H H OH Primary alcohol Aldehyde Carboxylic acid Figure 1 Ethanol oxidised to ethanal, and finally to ethanoic acid The equation for the oxidation of ethanol to the aldehyde ethanal is shown below. Note that the oxidising agent is shown as [O] – this simplifies the equation. CH3CH2OH + [O] }m CH3CHO + H2O When preparing aldehydes in the laboratory, you will need to distil the aldehyde from the reaction mixture as it is formed. This prevents the aldehyde from being oxidised further to a carboxylic acid. Key definition When making the carboxylic acid, the reaction mixture is usually heated under reflux before distilling the product off. -

Safety Data Sheet
SAFETY DATA SHEET Preparation Date: 06/23/2015 Revision Date: 10/27/2015 Revision Number: G2 1. IDENTIFICATION Product identifier Product code: P1282 Product Name: POTASSIUM DICHROMATE, CRYSTAL, REAGENT, ACS Other means of identification Synonyms: Bichromate of potash Chromium potassium oxide (K2Cr2O7) Dichromic acid dipotassium salt Dipotassium bichromate Dipotassium dichromate Dipotassium dichromium heptaoxide Iopezite Kaliumdichromat [German] Potassium bichromate Potassium chromate (K2Cr2O7) Potassium dichromate Potassium dichromate (K2(Cr2O7)) Potassium dichromate (K2Cr2O7) Potassium dichromate(VI) SRM 935a CAS #: 7778-50-9 RTECS # HX7680000 CI#: Not available Recommended use of the chemical and restrictions on use Recommended use: Analytical reagent. In tanning, printing, painting, electroplating and pyrotechnics. Uses advised against No information available Supplier: Spectrum Chemical Mfg. Corp 14422 South San Pedro St. Gardena, CA 90248 (310) 516-8000 Order Online At: https://www.spectrumchemical.com Emergency telephone number Chemtrec 1-800-424-9300 Contact Person: Martin LaBenz (West Coast) Contact Person: Ibad Tirmiz (East Coast) 2. HAZARDS IDENTIFICATION Classification This chemical is considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Product code: P1282 Product name: POTASSIUM 1 / 16 DICHROMATE, CRYSTAL, REAGENT, ACS Acute toxicity - Oral Category 2 Acute toxicity - Dermal Category 1 Acute toxicity - Inhalation (Gases) Category 2 Acute toxicity - Inhalation (Dusts/Mists) Category 2 Skin -
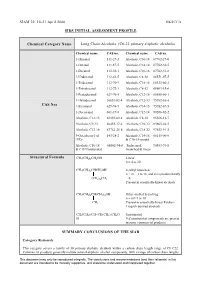
Long Chain Alcohols (C6-22 Primary Aliphatic Alcohols)
SIAM 22, 18-21 April 2006 UK/ICCA SIDS INITIAL ASSESSMENT PROFILE Chemical Category Name Long Chain Alcohols (C6-22 primary aliphatic alcohols) Chemical name CAS no. Chemical name CAS no. 1-Hexanol 111-27-3 Alcohols, C16-18 67762-27-0 1-Octanol 111-87-5 Alcohols, C14-18 67762-30-5 1-Decanol 112-30-1 Alcohols, C10-16 67762-41-8 1-Undecanol 112-42-5 Alcohols, C8-18 68551-07-5 1-Tridecanol 112-70-9 Alcohols, C14-16 68333-80-2 1-Tetradecanol 112-72-1 Alcohols, C6-12 68603-15-6 1-Pentadecanol 629-76-5 Alcohols, C12-16 68855-56-1 1-Hexadecanol 36653-82-4 Alcohols, C12-13 75782-86-4 CAS Nos 1-Eicosanol 629-96-9 Alcohols, C14-15 75782-87-5 1-Docosanol 661-19-8 Alcohols, C12-14 80206-82-2 Alcohols, C12-15 63393-82-8 Alcohols, C8-10 85566-12-7 Alcohols, C9-11 66455-17-2 Alcohols, C10-12 85665-26-5 Alcohols, C12-18 67762-25-8 Alcohols, C18-22 97552-91-5 9-Octadecen-1-ol 143-28-2 Alcohols, C14-18. 68155-00-0 (9Z)- & C16-18-unsatd Alcohols, C16-18 68002-94-8 Tridecanol, 90583-91-8 & C18 Unsaturated branched & linear Structural Formula CH3(CH2)nCH2OH Linear n = 4 to 20 CH3(CH2)nCHCH2OH 2-Alkyl branched n + m = 3 to 18, and m is predominantly (CH2)mCH3 = 0. Present in essentially-linear alcohols CH3(CH2)nCH(CH2)mOH Other-methyl branching n + m= 9 or 10 CH3 Present in essentially-linear Fischer- Tropsch derived alcohols CH3(CH2)7CH=CH(CH2)7CH2O Unsaturated H 9-Z unsaturated components are present in some commercial products. -

Transcription 12.01.12
Lecture 2B • 01/12/12 We covered three different reactions for converting alcohols into leaving groups. One was to turn an alcohol into an alkyl chloride, that was using thionyl chloride. Second reaction was using tosyl chloride; the primary difference between those two reactions is one of stereochemistry. An inversion of stereochemistry does occur if you use thionyl chloride, because it does affect the carbon-oxygen bond, but because forming a tosylate does not touch the carbon-oxygen bond, only the oxygen- hydrogen bond, there’s no change in stereochemistry there. We then saw phosphorus tribromide that reacts very similarly to the thionyl chloride; you get an alkyl bromide instead, but it also has inversion of configuration. The last reaction is not a new mechanism, it is just an Sn2 reaction, it’s called the Finkelstein reaction. Really this works, in a sense, off of Le Châtelier’s principle. In solution, in theory, sodium iodide can displace bromide, but sodium bromide can displace iodide, so you can have an Sn2 reaction that goes back and forth and back and forth and back and forth. Except, sodium iodide is somewhat soluble in acetone, while sodium chloride and sodium bromide are not. So, in fact, one of the things that we get out of this as a by-product is sodium bromide, which, again, is not soluble in acetone and therefore precipitates out and is no longer part of the reaction mixture, so there’s no reverse reaction possible. Because of this solubility trick, it allows this reaction to be pulled forward, which means you can get the alkyl iodide. -

Manufacturing of Potassium Permanganate Kmno4 This Is the Most Important and Well Known Salt of Permanganic Acid
Manufacturing of Potassium Permanganate KMnO4 This is the most important and well known salt of permanganic acid. It is prepared from the pyrolusite ore. It is prepared by fusing pyrolusite ore either with KOH or K2CO3 in presence of atmospheric oxygen or any other oxidising agent such as KNO3. The mass turns green with the formation of potassium manganate, K2MnO4. 2MnO2 + 4KOH + O2 →2K2MnO4 + 2H2O 2MnO2 + 2K2CO3 + O2 →2K2MnO4 + 2CO2 The fused mass is extracted with water. The solution is now treated with a current of chlorine or ozone or carbon dioxide to convert manganate into permanganate. 2K2MnO4 + Cl2 → 2KMnO4 + 2KCl 2K2MnO4 + H2O + O3 → 2KMnO4 + 2KOH + O2 3K2MnO4 + 2CO2 → 2KMnO4 + MnO2 + 2K2CO3 Now-a-days, the conversion is done electrolytically. It is electrolysed between iron cathode and nickel anode. Dilute alkali solution is taken in the cathodic compartment and potassium manganate solution is taken in the anodic compartment. Both the compartments are separated by a diaphragm. On passing current, the oxygen evolved at anode oxidises manganate into permanganate. At anode: 2K2MnO4 + H2O + O → 2KMnO4 + 2KOH 2- - - MnO4 → MnO4 + e + - At cathode: 2H + 2e → H2 Properties: It is purple coloured crystalline compound. It is fairly soluble in water. When heated alone or with an alkali, it decomposes evolving oxygen. 2KMnO4 → K2MnO4 + MnO2 + O2 4KMnO4 + 4KOH → 4K2MnO4 + 2H2O + O2 On treatment with conc. H2SO4, it forms manganese heptoxide via permanganyl sulphate which decomposes explosively on heating. 2KMnO4+3H2SO4 → 2KHSO4 + (MnO3)2SO4 + 2H2O (MnO3)2SO4 + H2O → Mn2O7 + H2SO4 Mn2O7 → 2MnO2 + 3/2O2 Potassium permanganate is a powerful oxidising agent. A mixture of sulphur, charcoal and KMnO4 forms an explosive powder. -

Chemical Compatibility Guide
Chemical Compatibility Guide Guide Applicable to the Following: PIG Portable Spill Containment Pool Guide Information This report is offered as a guide and was developed from information which, to the best of New Pig’s knowledge, was reliable and accurate. Due to variables and conditions of application beyond New Pig’s control, none of the data shown in this guide is to be construed as a guarantee, expressed or implied. New Pig assumes no responsibility, obligation, or liability in conjunction with the use or misuse of the information. PIG Spill Containment Pools are constructed from PVC-coated polyester fabric. The chemical resistance guide that follows shows the chemical resistance for the PVC layer only. This guide has been compiled to provide the user with general chemical resistance information. It does not reflect actual product testing. Ratings / Key or Ratings – Chemical Effect 1. Satisfactory to 72°F (22°C) 2. Satisfactory to 120°F (48°C) A = Excellent D = Severe Effect, not recommended for ANY use. B = Good — Minor Effect, slight corrosion or discoloration. N/A = Information not available. C = Fair — Moderate Effect, not recommended for continuous use. Softening, loss of strength, swelling may occur. Due to variables and conditions beyond our control, New Pig cannot guarantee that this product(s) will work to your satisfaction. To ensure effectiveness and your safety, we recommend that you conduct compatibility and absorption testing of your chemicals with this product prior to purchase. For additional questions or information, -

Perkin's Mauve: the History of the Chemistry
REFLECTIONS Perkin’s Mauve: The History of the Chemistry Andrew Filarowski Those of us who owe our living in part to the global dyestuff and chemical industry should pause today and remember the beginnings of this giant industry which started 150 years ago today with William Perkins’ discovery of mauveine whilst working in his home laboratory during the Easter holiday on April 28, 1856. Prior to this discovery, all textiles were dyed with natural dyestuffs and pigments. What did Perkin’s Reaction Entail? William Henry Perkin carried out his experiments at his home laboratory in the Easter break of 1856. He was trying to produce quinine (C20H24N2O2). This formula was known but not the structural formula. Because chemistry was in such an early stage of development Perkin thought that by simply balancing the masses (simple additive and subtractive chemistry) in an equation he would obtain the required compound. He therefore believed that if he took two allyltoluidine molecules, C10H13N, and oxidised them with three oxygen atoms (using potassium dichromate) he would get quinine (C20H24N2O2) and water. 2 (C10H13N) + 3O C20H24N2O2 + H2O It is unsurprising to us now but Perkin reported “that no quinine was formed, but only a dirty reddish brown precipitate.” However, he continued in his trials and decided to use aniline (C6H5NH2) and its sulphate, and to oxidise them using potassium dichromate. This produced a black precipitate that Perkin at first took to be a failed experiment, but he noticed on cleaning his equipment with alcohol that a coloured solution was obtained. Perkin’s Patent W H Perkin filed his patent on the 26th August 1856 for “Producing a new colouring matter for the dyeing with a lilac or purple color stuffs of silk, cotton, wool, or other materials.” (sic) Patent No. -

Expert Consensus Guidelines for Stocking of Antidotes in Hospitals That Provide Emergency Care
TOXICOLOGY/CONCEPTS Expert Consensus Guidelines for Stocking of Antidotes in Hospitals That Provide Emergency Care Richard C. Dart, MD, PhD From the Rocky Mountain Poison & Drug Center - Denver Health, Denver, CO (Dart, Heard, Schaeffer, Stephen W. Borron, MD, MS Bogdan, Alhelail, Buchanan, Hoppe, Lavonas, Mlynarchek, Phua, Rhyee, Varney, Zosel); Department of E. Martin Caravati, MD, MPH Surgery, University of Texas Health Sciences Center, San Antonio, TX (Borron); Division of Emergency Daniel J. Cobaugh, PharmD Medicine, Utah Poison Control Center, University of Utah Health Sciences Center, Salt Lake City, UT Steven C. Curry, MD (Caravati); ASHP Research and Education Foundation, Bethesda, MD (Cobaugh); Department of Jay L. Falk, MD Medical Toxicology and Banner Poison Control Center, Banner Good Samaritan Medical Center, Lewis Goldfrank, MD Phoenix, AZ (Curry); Department of Emergency Medicine, Orlando Regional Medical Center, University of Susan E. Gorman, PharmD, MS Florida, Orlando, FL (Falk); New York City Poison Center; New York University School of Medicine, New Stephen Groft, PharmD York, NY (Goldfrank); Division of Strategic National Stockpile, Centers for Disease Control and Kennon Heard, MD Prevention, Atlanta, GA (Gorman); Office of Rare Diseases Research, Bethesda, MD (Groft); Division of Ken Miller, MD, PhD Emergency Medicine, School of Medicine (Dart, Heard, Lavonas, Schaeffer) and Department of Kent R. Olson, MD Pharmaceutical Sciences, School of Pharmacy (Bogdan), University of Colorado Denver, Aurora, CO; Gerald O’Malley, DO Orange County Fire Authority and Orange County Health Care Agency Emergency Medical Services, Donna Seger, MD Irvine, CA, and National Association of EMS Physicians, Lenexa, KS, (Miller); University of California, Steven A. Seifert, MD San Francisco, and San Francisco Division, California Poison Control System, San Francisco, CA Marco L. -

Chemical Disinfectants | Disinfection & Sterilization Guidelines | Guidelines Library | Infection Control | CDC 3/19/20, 14:52
Chemical Disinfectants | Disinfection & Sterilization Guidelines | Guidelines Library | Infection Control | CDC 3/19/20, 14:52 Infection Control Chemical Disinfectants Guideline for Disinfection and Sterilization in Healthcare Facilities (2008) Alcohol Overview. In the healthcare setting, “alcohol” refers to two water-soluble chemical compounds—ethyl alcohol and isopropyl alcohol —that have generally underrated germicidal characteristics 482. FDA has not cleared any liquid chemical sterilant or high- level disinfectant with alcohol as the main active ingredient. These alcohols are rapidly bactericidal rather than bacteriostatic against vegetative forms of bacteria; they also are tuberculocidal, fungicidal, and virucidal but do not destroy bacterial spores. Their cidal activity drops sharply when diluted below 50% concentration, and the optimum bactericidal concentration is 60%–90% solutions in water (volume/volume) 483, 484. Mode of Action. The most feasible explanation for the antimicrobial action of alcohol is denaturation of proteins. This mechanism is supported by the observation that absolute ethyl alcohol, a dehydrating agent, is less bactericidal than mixtures of alcohol and water because proteins are denatured more quickly in the presence of water 484, 485. Protein denaturation also is consistent with observations that alcohol destroys the dehydrogenases of Escherichia coli 486, and that ethyl alcohol increases the lag phase of Enterobacter aerogenes 487 and that the lag phase e!ect could be reversed by adding certain amino acids. The bacteriostatic action was believed caused by inhibition of the production of metabolites essential for rapid cell division. Microbicidal Activity. Methyl alcohol (methanol) has the weakest bactericidal action of the alcohols and thus seldom is used in healthcare 488. The bactericidal activity of various concentrations of ethyl alcohol (ethanol) was examined against a variety of microorganisms in exposure periods ranging from 10 seconds to 1 hour 483.