An Adiabatic Saturation Psychrometer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplemental Information 1: System Assumptions
Electronic Supplementary Material (ESI) for Lab on a Chip This journal is © The Royal Society of Chemistry 2011 Supplemental Information 1: System Assumptions Starting from the mass transfer equation: ∂C 2 + υ ⋅ ∇C = D∇ C + R ∂t (a) Distance between the droplet and air channels, O(10‐4) m is much smaller than the distance between the droplet channel PDMS device boundary O(10‐2) m, so little € mass transfer is out of the device and most mass transfer is into the air channel. (b) Assume the oil provides no resistance as compared to the PDMS. (c) Assume psuedo‐steady state since the PDMS boundaries and concentration boundary conditions are fixed: ∂C = 0 ∂t (d) No convection € υ = 0 (e) No reaction € R = 0 (f) Since the oil provides little resistance to mass transfer, the end of the droplets can be assumes as a constant value with surface area equal to the height of the droplet, h, multiplied by the width of the droplet (g) The concentration out the top is a function of the distance between droplet and air channels and will related to mass transfer out the sides for fixed droplet/air separations. This mass transfer can be represented by multiplying by an effective area. Therefore the system is simplified to one dimension. ∂ 2C = 0 ∂x 2 BC1 C(x = 0) = Csat,PDMS € Csat,PDMS BC2 C(x = d) = Cair = HCair Csat,air € (Csat,PDMS − HCair ) C = Csat,PDMS − x d € dC C − HC J = −D = D ( sat,PDMS air ) dx d € € Electronic Supplementary Material (ESI) for Lab on a Chip This journal is © The Royal Society of Chemistry 2011 Supplemental Information 2: Droplet Change This derivation and model is derived assuming an approximate rectangular block shaped droplet with constant fluxes (Jtop, Jend, Jside) out of each the top, the ends and the side of the drop (Atop, Aend, Aside) in Equation S1. -

5892 Cisco Category: Standards Track August 2010 ISSN: 2070-1721
Internet Engineering Task Force (IETF) P. Faltstrom, Ed. Request for Comments: 5892 Cisco Category: Standards Track August 2010 ISSN: 2070-1721 The Unicode Code Points and Internationalized Domain Names for Applications (IDNA) Abstract This document specifies rules for deciding whether a code point, considered in isolation or in context, is a candidate for inclusion in an Internationalized Domain Name (IDN). It is part of the specification of Internationalizing Domain Names in Applications 2008 (IDNA2008). Status of This Memo This is an Internet Standards Track document. This document is a product of the Internet Engineering Task Force (IETF). It represents the consensus of the IETF community. It has received public review and has been approved for publication by the Internet Engineering Steering Group (IESG). Further information on Internet Standards is available in Section 2 of RFC 5741. Information about the current status of this document, any errata, and how to provide feedback on it may be obtained at http://www.rfc-editor.org/info/rfc5892. Copyright Notice Copyright (c) 2010 IETF Trust and the persons identified as the document authors. All rights reserved. This document is subject to BCP 78 and the IETF Trust's Legal Provisions Relating to IETF Documents (http://trustee.ietf.org/license-info) in effect on the date of publication of this document. Please review these documents carefully, as they describe your rights and restrictions with respect to this document. Code Components extracted from this document must include Simplified BSD License text as described in Section 4.e of the Trust Legal Provisions and are provided without warranty as described in the Simplified BSD License. -
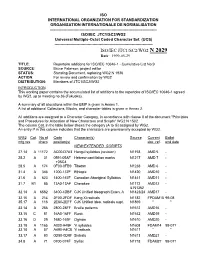
ISO/IEC JTC1/SC2/WG2 N 2029 Date: 1999-05-29
ISO INTERNATIONAL ORGANIZATION FOR STANDARDIZATION ORGANISATION INTERNATIONALE DE NORMALISATION --------------------------------------------------------------------------------------- ISO/IEC JTC1/SC2/WG2 Universal Multiple-Octet Coded Character Set (UCS) -------------------------------------------------------------------------------- ISO/IEC JTC1/SC2/WG2 N 2029 Date: 1999-05-29 TITLE: Repertoire additions for ISO/IEC 10646-1 - Cumulative List No.9 SOURCE: Bruce Paterson, project editor STATUS: Standing Document, replacing WG2 N 1936 ACTION: For review and confirmation by WG2 DISTRIBUTION: Members of JTC1/SC2/WG2 INTRODUCTION This working paper contains the accumulated list of additions to the repertoire of ISO/IEC 10646-1 agreed by WG2, up to meeting no.36 (Fukuoka). A summary of all allocations within the BMP is given in Annex 1. A list of additional Collections, Blocks, and character tables is given in Annex 2. All additions are assigned to a Character Category, in accordance with clause II of the document "Principles and Procedures for Allocation of New Characters and Scripts" WG2 N 1502. The column Cat. in the table below shows the category (A to G) assigned by WG2. An entry P in this column indicates that the characters are provisionally accepted by WG2. WG2 Cat. No of Code Character(s) Source Current Ballot mtg.res chars position(s) doc. ref. end date NEW/EXTENDED SCRIPTS 27.14 A 11172 AC00-D7A3 Hangul syllables (revision) N1158 AMD 5 - 28.2 A 31 0591-05AF Hebrew cantillation marks N1217 AMD 7 - +05C4 28.5 A 174 0F00-0FB9 Tibetan N1238 AMD 6 - 31.4 A 346 1200-137F Ethiopic N1420 AMD10 - 31.6 A 623 1400-167F Canadian Aboriginal Syllabics N1441 AMD11 - 31.7 B1 85 13A0-13AF Cherokee N1172 AMD12 - & N1362 32.14 A 6582 3400-4DBF CJK Unified Ideograph Exten. -

MUFI Character Recommendation V. 3.0: Alphabetical Order
MUFI character recommendation Characters in the official Unicode Standard and in the Private Use Area for Medieval texts written in the Latin alphabet ⁋ ※ ð ƿ ᵹ ᴆ ※ ¶ ※ Part 1: Alphabetical order ※ Version 3.0 (5 July 2009) ※ Compliant with the Unicode Standard version 5.1 ____________________________________________________________________________________________________________________ ※ Medieval Unicode Font Initiative (MUFI) ※ www.mufi.info ISBN 978-82-8088-402-2 ※ Characters on shaded background belong to the Private Use Area. Please read the introduction p. 11 carefully before using any of these characters. MUFI character recommendation ※ Part 1: alphabetical order version 3.0 p. 2 / 165 Editor Odd Einar Haugen, University of Bergen, Norway. Background Version 1.0 of the MUFI recommendation was published electronically and in hard copy on 8 December 2003. It was the result of an almost two-year-long electronic discussion within the Medieval Unicode Font Initiative (http://www.mufi.info), which was established in July 2001 at the International Medi- eval Congress in Leeds. Version 1.0 contained a total of 828 characters, of which 473 characters were selected from various charts in the official part of the Unicode Standard and 355 were located in the Private Use Area. Version 1.0 of the recommendation is compliant with the Unicode Standard version 4.0. Version 2.0 is a major update, published electronically on 22 December 2006. It contains a few corrections of misprints in version 1.0 and 516 additional char- acters (of which 123 are from charts in the official part of the Unicode Standard and 393 are additions to the Private Use Area). -

1 Symbols (2286)
1 Symbols (2286) USV Symbol Macro(s) Description 0009 \textHT <control> 000A \textLF <control> 000D \textCR <control> 0022 ” \textquotedbl QUOTATION MARK 0023 # \texthash NUMBER SIGN \textnumbersign 0024 $ \textdollar DOLLAR SIGN 0025 % \textpercent PERCENT SIGN 0026 & \textampersand AMPERSAND 0027 ’ \textquotesingle APOSTROPHE 0028 ( \textparenleft LEFT PARENTHESIS 0029 ) \textparenright RIGHT PARENTHESIS 002A * \textasteriskcentered ASTERISK 002B + \textMVPlus PLUS SIGN 002C , \textMVComma COMMA 002D - \textMVMinus HYPHEN-MINUS 002E . \textMVPeriod FULL STOP 002F / \textMVDivision SOLIDUS 0030 0 \textMVZero DIGIT ZERO 0031 1 \textMVOne DIGIT ONE 0032 2 \textMVTwo DIGIT TWO 0033 3 \textMVThree DIGIT THREE 0034 4 \textMVFour DIGIT FOUR 0035 5 \textMVFive DIGIT FIVE 0036 6 \textMVSix DIGIT SIX 0037 7 \textMVSeven DIGIT SEVEN 0038 8 \textMVEight DIGIT EIGHT 0039 9 \textMVNine DIGIT NINE 003C < \textless LESS-THAN SIGN 003D = \textequals EQUALS SIGN 003E > \textgreater GREATER-THAN SIGN 0040 @ \textMVAt COMMERCIAL AT 005C \ \textbackslash REVERSE SOLIDUS 005E ^ \textasciicircum CIRCUMFLEX ACCENT 005F _ \textunderscore LOW LINE 0060 ‘ \textasciigrave GRAVE ACCENT 0067 g \textg LATIN SMALL LETTER G 007B { \textbraceleft LEFT CURLY BRACKET 007C | \textbar VERTICAL LINE 007D } \textbraceright RIGHT CURLY BRACKET 007E ~ \textasciitilde TILDE 00A0 \nobreakspace NO-BREAK SPACE 00A1 ¡ \textexclamdown INVERTED EXCLAMATION MARK 00A2 ¢ \textcent CENT SIGN 00A3 £ \textsterling POUND SIGN 00A4 ¤ \textcurrency CURRENCY SIGN 00A5 ¥ \textyen YEN SIGN 00A6 -

International Standard Iso/Iec 10646-1
This preview is downloaded from www.sis.se. Buy the entire standard via https://www.sis.se/std-615259 INTERNATIONAL ISO/IEC STANDARD 10646-1 First edition 1993-05-01 AMENDMENT 18 1999-07-15 Information technology — Universal Multiple-Octet Coded Character Set (UCS) — Part 1: Architecture and Basic Multilingual Plane AMENDMENT 18: Symbols and other characters Technologies de l'information — Jeu universel de caractères codés à plusieurs octets — Partie 1: Architecture et table multilingue AMENDEMENT 18: Symboles et autres caractères bc Reference number ISO/IEC 10646-1:1993/Amd.18:1999(E) This preview is downloaded from www.sis.se. Buy the entire standard via https://www.sis.se/std-615259 ISO/IEC 10646-1:1993/Amd.18:1999(E) Foreword ISO (the International Organization for Standardization) and IEC (the Inter- national Electrotechnical Commission) form the specialized system for worldwide standardization. National bodies that are members of ISO or IEC participate in the development of International Standards through technical committees established by the respective organization to deal with particular fields of technical activity. ISO and IEC technical committees collaborate in fields of mutual interest. Other international organizations, governmental and non-governmental, in liaison with ISO and IEC, also take part in the work. In the field of information technology, ISO and IEC have established a joint technical committee, ISO/IEC JTC 1. Draft International Standards adopted by the joint technical committee are circulated to national bodies for voting. Publication as an International Standard requires approval by at least 75 % of the national bodies casting a vote. Amendment 18 to ISO/IEC 10646-1:1993 was prepared by Joint Technical Committee ISO/IEC JTC 1, Information technology, Subcommittee SC 2, Coded character sets. -

Page De Garde
N° d‘ordre : Université Kasdi Merbah Ouargla N° de série : FACULTE DES SCIENCES ET SCIENCES DE L'INGENIEUR DEPARTEMENT DE GENIE DES PROCEDES Mémoire Présenté pour l'obtention du diplôme de MAGISTER en Génie des Procédés Option : Génie Chimique Par : Lakhdari Abdelhakim TTThèmeThème Contribution à l’étude des réacteurs à lits fluidisés : Modélisation du comportement hydrodynamique et thermique du réacteur Soutenu publiquement le : 17 / 05 / 2006 devant le jury composé de : Mr. Ladjel Segni M.C Université de Ouargla Président Mr. Salah Saouli M.C Université de Ouargla Examinateur Mr. Noureddine Settou M.C Université de Ouargla Examinateur Mr. Salah Dounit M.C Université de Ouargla Rapporteur Remerciement Merci à Dieu pour tous ce qu’il m’a offert. Je tiens à remercier mon directeur de mémoire, Dr Salah DOUNI , Maître de Conférences à l’université de Ouargla, qu’il veuille bien trouver ici l’expression de mes reconnaissances. Je tiens le remercier très chaleureusement pour le soin qu’il m’a apporté à la préparation de ce mémoire, ainsi que pour son attention qui m’a accordé afin de mener à terme ce travail. Je tiens à remercier et Je suis très reconnaissant au président du jury monsieur Dr Segni LADJEL, Maître de Conférences à l’université de Ouargla, qui m’a fait l’honneur d’avoir bien accepter de juger mon travail. Comme je le remercie pour l’aide qui m’a offert durant toute la période du travail. Je remercier très infiniment mon ancien formateur au cycle de la graduation monsieur Dr Salah SAOULI , Maître de Conférences à l’université de Ouargla, de m’avoir fait l’honneur d’accepter de faire partie de la commission d’examen de ce mémoire. -

Latin Extended-B Range: 0180–024F
Latin Extended-B Range: 0180–024F This file contains an excerpt from the character code tables and list of character names for The Unicode Standard, Version 14.0 This file may be changed at any time without notice to reflect errata or other updates to the Unicode Standard. See https://www.unicode.org/errata/ for an up-to-date list of errata. See https://www.unicode.org/charts/ for access to a complete list of the latest character code charts. See https://www.unicode.org/charts/PDF/Unicode-14.0/ for charts showing only the characters added in Unicode 14.0. See https://www.unicode.org/Public/14.0.0/charts/ for a complete archived file of character code charts for Unicode 14.0. Disclaimer These charts are provided as the online reference to the character contents of the Unicode Standard, Version 14.0 but do not provide all the information needed to fully support individual scripts using the Unicode Standard. For a complete understanding of the use of the characters contained in this file, please consult the appropriate sections of The Unicode Standard, Version 14.0, online at https://www.unicode.org/versions/Unicode14.0.0/, as well as Unicode Standard Annexes #9, #11, #14, #15, #24, #29, #31, #34, #38, #41, #42, #44, #45, and #50, the other Unicode Technical Reports and Standards, and the Unicode Character Database, which are available online. See https://www.unicode.org/ucd/ and https://www.unicode.org/reports/ A thorough understanding of the information contained in these additional sources is required for a successful implementation. -

ISO/IEC JTC 1/SC 2 N 3188 Date: 1998-10-22
ISO/IEC JTC 1/SC 2 N 3188 Date: 1998-10-22 Replaces SC 2 N 3002 ISO/IEC JTC 1/SC 2 CODED CHARACTER SETS SECRETARIAT: JAPAN (JISC) DOC TYPE: Text for FDAM ballot TITLE: Revised Text of ISO/IEC 10646-1/FPDAM 18, Universal Multiple-Octet Coded Character set (UCS) -- Part 1: Architecture and Basic Multilingual Plane -- AMENDMENT 18: Symbols and Others SOURCE: Project Editor PROJECT: JTC 1.02.18.01.18 STATUS: In accordance with Resolution M35.01 adopted at the 35th meeting of SC 2/WG 2 held in London, UK, 1998-09-21/25, this document has been prepared by Project Editor. It is submitted to ITTF for a two-month FDAM ballot. ACTION ID: ITTF DUE DATE: -- DISTRIBUTION: P, O and L Members of ISO/IEC JTC 1/SC 2 WG Conveners and Secretariats Secretariat, ISO/IEC JTC 1 ISO/IEC ITTF NO. OF PAGES: 5 ACCESS LEVEL: Defined WEB ISSUE #: 032 Contact: Secretariat ISO/IEC JTC 1/SC 2 - Toshiko KIMURA IPSJ/ITSCJ (Information Processing Society of Japan/Information Technology Standards Commission of Japan)* Room 308-3, Kikai-Shinko-Kaikan Bldg., 3-5-8, Shiba-Koen, Minato-ku, Tokyo 105-0011 JAPAN Tel: +81 3 3431 2808; Fax: +81 3 3431 6493; E-mail: [email protected]; http://www.dkuug.dk/jtc1/sc2 *A Standard Organization accredited by JISC © ISO/IEC FDAM for ISO/IEC 10646-1: 1993/Amd. 18: 1999 (E) Information technology — Universal Multiple-Octet Coded Character Set (UCS) — Part 1: Architecture and Basic Multilingual Plane AMENDMENT 18: Symbols and other characters 1. -

Editors' Introduction
Editors’ Introduction This is the 14th issue of Tolkien Studies, the first refereed journal solely devoted to the scholarly study of the works of J.R.R. Tolkien. As edi- tors, our goal is to publish excellent scholarship on Tolkien as well as to gather useful research information, reviews, notes, documents, and bibliographical material. All articles have been subject to anonymous, external review as well as receiving a positive judgment by the Editors. In the cases of articles by individuals associated with the journal in any way, each article had to receive at least two positive evaluations from two dif fer ent outside reviewers. Reviewer comments wer e anonymously conveyed to the au- thors of the articles. The Editors agreed to be bound by the recommen- dations of the outside referees. Although they are solicited and edited by the editors, book reviews represent the judgments of the individ- ual reviewers, not Tolkien Studies. Michael D. C. Drout Verlyn Flieger David Bratman 1 Acknowl edgments The Editors would like to thank Wheaton College, Norton, MA and Wheaton Research Partners for their support. Thanks also to Rowan Lowell, Paula Smith- MacDonald, Vaughn Howland, Raquel D’Oyen, and Berni Phillips, and also to West V irginia University Press, Derek Krissoff, Than Saffel, and Jason Gosnell. Fi nally, we acknowledge a special debt of gratitude to our anony- mous, outside reviewers who with their collegial ser vice contribute so much to Tolkien Studies. 2 In Memoriam Tolkien Studies notes the passing of Richard L(awrence) Purtill (1931– 2016), who died on December 4, 2016. -

MUFI Character Recommendation V. 4.0: Alphabetical Order
MUFI character recommendation Characters in the official Unicode Standard and in the Private Use Area for Medieval texts written in the Latin alphabet ⁋ ※ ð ƿ ᵹ ᴆ ※ ¶ ※ Part 1: Alphabetical order ※ Version 4.0 (22 December 2015) ※ Compliant with the Unicode Standard version 8.0 ____________________________________________________________________________________________________________________ ※ Medieval Unicode Font Initiative (MUFI) ※ www.mufi.info http://hdl.handle.net/1956/10699 ※ Characters on shaded background belong to the Private Use Area. Please read the introduction p. 11 carefully before using any of these characters. MUFI character recommendation ※ Part 1: alphabetical order version 4.0 p. 2 / 168 Editor Odd Einar Haugen, University of Bergen, Norway. Background Version 1.0 of the MUFI recommendation was published electronically and in hard copy on 8 December 2003. It was the result of an almost two-year-long electronic discussion within the Medieval Unicode Font Initiative (http://www.mufi.info), which was established in July 2001 at the International Medie- val Congress in Leeds. Version 1.0 contained a total of 828 characters, of which 473 characters were selected from various charts in the official part of the Unicode Standard and 355 were located in the Private Use Area. Version 1.0 of the recommendation was compliant with the Unicode Standard version 4.0. Version 2.0 was a major update, published electronically on 22 December 2006. The net addition in this version was 498 characters, making a total of 1326 characters. This version of the recommendation was compliant with the Unicode Standard version 5.0. Version 3.0 was another major update, published electronically on 24 June 2009. -

[email protected] MARK STEELE May 3, 2012 (416) 359-4641
MARK STEELE (416) 359-4641 [email protected] May 3, 2012 Tiberiu Stoichita (416) 359-4684 [email protected] Global Group vs. S&P Global 1200 Index Advertising 21% Pipelines 30% Truk&Lease 19% Auto Manu 11% Banks -10% Hm Furnish -22% Coal -29%a Alt Energy -69% Hme Build 21% Lodging 29% Biotech 15% ShipBuild 8% Div Financ -7% Mining -21% Airlines -28% Electric -26% SemiCon 17% Computers 23% Apparel 14% Software 7% Leisure Time -6% DryShips -19% Toys/Game -26% Telecom -22% Media 12%a Gaming 18% FoodServ 13% Real Estate 7% Mch Cnst/Mng -5% Off/BusEquip -17% Elec Compon -22% Environ Ctrl -20% Com Serv 7% Agriculture 9% Tools 7% Storage -5% Iron/Steel -17% Engin&Const -19% Uranium -14% Aero/Def 6% InvestCo 6% HcareServ 5% Pharma -5% Electronics -13% Textiles -18% Misc Mfg 6% Auto Parts 4% OfceFurn -4% Water -13%a Gas -17% Retail 4% Mtl Fab -3% Cosm/Pers -9% O&G Serv -16% Forest Pdcts 2% Save&Loan -2% Mch Divers -9% Food -12% Bld Mat 1% Dist/Wsale -2% Houseware -8% Transport -8% Insurance 1% Entertain -2%a Chemicals 0% Beverages -1%a Oil&Gas -1% HcareProd -1%a Pkg&Contain 0% Hm Products 0%a Page 2 TREND CHANGES vs S&P Global 1200 Index Accelerating Uptrends DANG InetRtl 124% SCSS HmFrnsh 112% H27 HK Cas&Gam 78% «CSTR SpCmSrv 75% «AGS LN Advert 56% «BOX TrdgDist 44% «XLS Aero&Def 39% «HAFC RegionBnk 60% «GCARSOA1 MMIndCongl 31% HLS HCFacility 29% «NAL EnviroServ 22% BRKR LfSciSrv 23% WG.