Stopping an Invisible Epidemic: Legal Issues in the Provision of Naloxone to Prevent Opioid Overdose
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Text Bruce Hainley
Kouros by Yves Saint Laurent 1. Brad Renfro, Skeet Ulrich, Axl Rose, Leif Garrett—I’m listing those things or people I find immediately identifiable in this Hawkins painting of 2017 that I’m looking at in a fairly hi-res digital reproduction, while sitting under a canopy in the noonday sun of Cornino, clear across the entire island of Sicily from Taormina— two different Morandi still lifes (at the top and near the bottom of the painting), numerous b/w reproductions of various Greco-Roman and/or Greco-Roman-like statuary (a few Hercules-es, some hermaphrodites), a vivid pink and yellow de Kooning, Richard Lindner’s Ice (1966), a particular guy with a prominent red kanji or ideogram tattoo on his left pectoral whose name it kills me I can’t recall at the moment and with/by whom Hawkins was obsessed/distracted for a year or more, a few Japanese male models ditto (i.e., the artist was obsessed/distracted with/by them, too, and it kills me I can’t think of their names [not Daisuke Ueda, not Seijo, not Osamu Mukai]), an Egon Schiele self-portrait (?), perhaps a bit of a Rudolf Schwarzkogler perf, Lon Chaney (maybe Bela Lugosi) on an elaborate Hollywood-set staircase as Dracula. Walking down an ersatz West Village street, Tom Cruise has fallen into daydreaming again, triggered by a man and woman kissing deeply in front of a gaudy shop (windows trimmed in neon, lace, Christmas lights); he flashes on Nicole and her fantasy naval officer going at it: this time she’s almost naked, his strong hand fingers her pussy. -

NAME E-Book 2012
THE HISTORY OF THE NAME National Association of Medical Examiners Past Presidents History eBook 2012 EDITION Published by the Past Presidents Committee on the Occasion of the 46th Annual Meeting at Baltimore, Maryland Preface to the 2012 NAME History eBook The Past Presidents Committee has been continuing its effort of compiling the NAME history for the occasion of the 2016 NAME Meeting’s 50th Golden Anniversa- ry Meeting. The Committee began collecting historical materials and now solicits the histories of individual NAME Members in the format of a guided autobiography, i.e. memoir. Seventeen past presidents have already contributed their memoirs, which were publish in a eBook in 2011. We continued the same guided autobiography format for compiling historical ma- terial, and now have additional memoirs to add also. This year, the book will be combined with the 2011 material, and some previous chapters have been updated. The project is now extended to all the NAME members, who wish to contribute their memoirs. The standard procedure is also to submit your portrait with your historical/ memoir material. Some of the memoirs are very short, and contains a minimum information, however the editorial team decided to include it in the 2012 edition, since it can be updated at any time. The 2012 edition Section I – Memoir Series Section II - ME History Series – individual medical examiner or state wide system history Presented in an alphabetic order of the name state Section III – Dedication Series - NAME member written material dedicating anoth- er member’s contributions and pioneer work, or newspaper articles on or dedicated to a NAME member Plan for 2013 edition The Committee is planning to solicit material for the chapters dedicated to specifi- cally designated subjects, such as Women in the NAME, Standard, Inspection and Accreditation Program. -

WHAT I LEARNED at the MOVIES ABOUT LEGAL ETHICS and PROFESSIONALISM by Anita Modak-Truran
WHAT I LEARNED AT THE MOVIES ABOUT LEGAL ETHICS AND PROFESSIONALISM By Anita Modak-Truran HOW I GOT HERE I’ve been fortunate. I practice law. I make movies. I write about both. I took up my pen and started writing a film column for The Clarion-Ledger, a Gannett-owned newspaper, back in the late 90s, when I moved from Chicago, Illinois, to Jackson, Mississippi. (It was like a Johnny Cash song… “Yeah, I’m going to Jackson. Look out Jackson Town….”) I then turned my pen to writing for The Jackson Free Press, an indie weekly newspaper, which provided me opportunities to write about indie films and interesting people. I threw down the pen, as well as stopped my public radio movie reviews and the television segment I had for an ABC affiliate, when I moved three years ago from Jackson to Nashville to head Butler Snow’s Entertainment and Media Industry Group. During my journey weaving law and film together in a non-linear direction with no particular destination, I lived in the state where a young lawyer in the 1980s worked 60 to 70 hours a week at a small town law practice, squeezing in time before going to the office and during courtroom recesses to work on his first novel. John Grisham writes that he would not have written his first book if he had not been a lawyer. “I never dreamed of being a writer. I wrote only after witnessing a trial.” See http://www.jgrisham.com/bio/ (last accessed January 24, 2016). My law partners at Butler Snow have stories about the old days when Mr. -
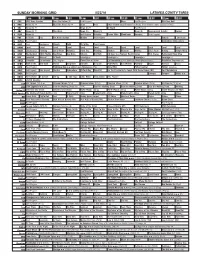
Sunday Morning Grid 5/22/16 Latimes.Com/Tv Times
SUNDAY MORNING GRID 5/22/16 LATIMES.COM/TV TIMES 7 am 7:30 8 am 8:30 9 am 9:30 10 am 10:30 11 am 11:30 12 pm 12:30 2 CBS CBS News Sunday Face the Nation (N) Paid Program Boss Paid Program PGA Tour Golf 4 NBC News (N) Å Meet the Press (N) Å News Paid 2016 French Open Tennis First Round. From Roland Garros Stadium in Paris. 5 CW News (N) Å News (N) Å In Touch Paid Program 7 ABC News (N) Å This Week News (N) News (N) News (N) Supervisorial Debate Explore 9 KCAL News (N) Joel Osteen Schuller Pastor Mike Woodlands Amazing Paid Program 11 FOX In Touch Paid Fox News Sunday Midday Paid Program FabLab I Love Lucy 13 MyNet Paid Program Somewhere Slow (2013) 18 KSCI Paid Hormones Church Faith Dr. Willar Paid Program 22 KWHY Local Local Local Local Local Local Local Local Local Local Local Local 24 KVCR Landscapes Painting Joy of Paint Wyland’s Paint This Painting Kitchen Mexican Martha Ellie’s Real Baking Simply Ming 28 KCET Wunderkind 1001 Nights Bug Bites Bug Bites Edisons Biz Kid$ 30 Days to a Younger Heart-Masley Rick Steves Retirement Road Map 30 ION Jeremiah Youssef In Touch Leverage Å Leverage Å Leverage Å Leverage Å 34 KMEX Conexión En contacto Paid Program Como Dice el Dicho La Comadrita (1978, Comedia) María Elena Velasco. República Deportiva (N) 40 KTBN Walk in the Win Walk Prince Carpenter Schuller In Touch PowerPoint It Is Written Pathway Super Kelinda Jesse 46 KFTR Paid Program Firehouse Dog ›› (2007) Josh Hutcherson. -

HOW to ROB a BANK ..And 10 Tips to Actually Get Away with It
Rick Lashbrook Films Williamsburg Media Cult & Villa Entertainment present HOW TO ROB A BANK ..and 10 tips to actually get away with it Sta rr in g Nic k Stah l, E ri ka Chr is tens en & Ga vin Ro ss dal e 81:08 R unni ng Ti me, Co lor (US, 20 07) Domestic Sales International Sales Shaun Redick Gary Hamilton & Lina Marrone The Collective/Collective Films Incorporated (CFI) Arclight Films International 9100 Wilshire Blvd. 9229 Sunset Blvd. Suite 700 West Suite 705 Beverly Hills, CA 90212 Los Angeles, CA 90069 310-888-1511 phone 310-777-8855 phone 310-888-1555 facsimile 310-777-8882 facsimile www.thecollective-la.com www.archlightfilms.com 1 of 23 CONTENTS PRODUCTION NOTES Story Logline ..................................................................................................... p 4 Story Synopsis ................................................................................................... p 4 The Screenplay & Background............................................................................. p 4 Principal Photography......................................................................................... p 5 Cast Credits ....................................................................................................... p 7 CAST BIOGRAPHIES Nick Stahl.......................................................................................................... p 8 Erika Christensen ............................................................................................... p 8 Gavin Rossdale ................................................................................................. -

Schedule List
Virginia Film Festival Films of 2019 2019 Late Night Wrap Party Three Notch’d Craft Kitchen & Brewery Saturday October 26 10:00 PM - 2:00 AM 21+ Event Before the credits roll on the 2019 Festival, join us at the Late Night Wrap Party for an unforgettable evening. Enjoy delicious local beer and savory snacks provided by Three Notch’d Craft Kitchen & Brewery and refreshing Bold Rock Hard Cider. And don’t miss one of your last chances to sample Three Notch’d Brewery’s special edition VAFF beer brewed specifically for our Film Festival season! Dance to rocking tunes and try out the MoxBox social photo booth. Mingle with filmmakers and fellow movie fans as you bask in the excitement and energy of VAFF. Presented by the Virginia Film Office and Three Notch’d Brewing Company 2019 Opening Night Gala The Jefferson Theater Wednesday October 23 9:30 PM - 12:00 AM 21+ Event Join us for the start of the Virginia Film Festival at the Opening Night Gala. The Gala brings together visiting stars and Festival patrons in celebration of the magic and beauty of film. Dance to the delightful sound of Kool Kats Lite, savor hors d’oeuvres from Harvest Moon Catering, take home memories from the evening with the MoxBox social photo booth, and enjoy delicious local beverages as we toast the Festival weekend to come. Presented by Bank of America Supported by Harvest Moon Catering and The AV Company Event Partner – Bold Rock Hard Cider 2040 Newcomb Hall Theatre Sunday October 27 2:00 PM - 4:00 PM Director: Damon Gameau Featuring: Damon Gameau, Eva Lazzaro, Zoë Gameau What will our planet look like in the year 2040? And more importantly, can we do anything to make a difference in our future? Director Damon Gameau argues the answer is “yes” in this idealistic and hopeful documentary that imagines the year 2040 as a brighter and better world, despite concerns about the planet’s declining health. -

Chris Helcermanas-Benge
Chris Helcermanas-Benge Photographer 6588 Wellington Avenue, West Vancouver, BC V7W 2H9 1-604-921-9882 Email - [email protected] http://www.imdb.me/chrish-b CHRIS HELCERMANAS-BENGE is recognized for his creative and innovative photography. Dual Canadian / American citizenship and EEC work visa enable him to shoot freely throughout the world. 36 years of producing stills for film, industry and government has taken Chris to 72 countries. He has been the photographer 200+ film projects; covered 3 wars, 11 combat zones, numerous world events; won many awards, and been published worldwide. He is a member of IATSE local 600 (USA), founding member of IATSE Locals 667 and 669 (Canada), and the BC. and Yukon Council of Film Unions and serving twice as president of 669. Chris H-B works well with talent, creative, and production to generate the finest images for the client. Chris has a BA in Military History, MA in International Affairs and a diploma in Fine Arts. His Military service included Southeast Asia, Africa, Greece, Germany, Turkey, Africa, the Pentagon, and the Middle East. He is a licensed pilot; a Padi certified diver, and parachutist, trained in desert, jungle, and mountain survival. He is an experienced sailor, a kayaker, mountaineer, Bon vivant, lover of fine wines and avid cross-country skier Chris is a member of C.A.P.I.C., The Overseas Press Club, The American Society of Media Photographers, National Press Photographer's Association, Combat Photographer's Association, I.A.T.S.E. (roster member US Local 600, Canadian Locals 667 and 669). -

Eastern Illinois University the Keep
Eastern Illinois University The Keep January 1999 1-15-1999 Daily Eastern News: January 15, 1999 Eastern Illinois University Follow this and additional works at: http://thekeep.eiu.edu/den_1999_jan Recommended Citation Eastern Illinois University, "Daily Eastern News: January 15, 1999" (1999). January. 5. http://thekeep.eiu.edu/den_1999_jan/5 This Article is brought to you for free and open access by the 1999 at The Keep. It has been accepted for inclusion in January by an authorized administrator of The Keep. For more information, please contact [email protected]. 26˚ The Daily Friday Flurries 16˚ January 15, 1999 Inside Eastern Sports Entertainment www.den.eiu.edu Skyhawks’ Eastern Illinois University year in review Charleston, Ill. 61920 air attack The Verge staff gives its top Vol. 84, No. 80 Tennessee Martin’s strong 12 pages ten on everything from music shooting performance to movies. News grounded the Panthers 83-75. The Verge, section B “Tell the truth and don’t be afraid.” Story on Page 8A State of university ‘Drug of choice’ put on hold Police: Marijuana Jorns declines to give familiarize herself with annual address, Surles the campus use down at Eastern given opportunity and would be in a better By Joe Sanner By Meghan McMahon position to Senior reporter Administration editor convey her thoughts and ot, Mary Jane, cheeba cheeba, weed – Eastern President David aspirations whatever you call it, law enforcement Jorns has declined to give the President Jorns for the univer- officials throughout Charleston and the annual State of the University sity,” he said. PColes County area say they agree marijua- address before retiring in early Faculty Senate Chair James na is “the drug of choice” for college students and March. -

Four Star Films, Box Office Hits, Indies and Imports, Movies A
Four Star Films, Box Office Hits, Indies and Imports, Movies A - Z FOUR STAR FILMS Top rated movies and made-for-TV films airing the week of the week of Sept 5 - 11, 2021 The Adventures of Robin Hood (1938) TCM Mon. 9 a.m. Alien (1979) AMC Tues. 9:30 a.m. AMC Tues. 12:26 p.m. Aliens (1986) AMC Tues. Noon AMC Wed. 9 a.m. Butch Cassidy and the Sundance Kid (1969) EPIX Sun. 4:15 p.m. Chinatown (1974) Showtime Tues. 7:45 a.m. Cinema Paradiso (1988) TCM Mon. 5 p.m. The Crying Game (1992) TMC Tues. 3:35 a.m. The Dark Knight (2008) Paramount Sat. 3:30 p.m. Paramount Sat. 10:30 p.m. East of Eden (1955) TCM Tues. 5:30 a.m. Forrest Gump (1994) AMC Thur. 8 p.m. AMC Fri. 5 p.m. The Good, the Bad and the Ugly: Restored Version (1967) TCM Mon. 7:15 p.m. Goodfellas (1990) AMC Tues. 8 p.m. AMC Wed. 5 p.m. The Graduate (1967) TCM Wed. 5 p.m. The Hustler (1961) TCM Sat. 7:30 p.m. The Last Picture Show (1971) TCM Sat. 11:45 p.m. The Little Mermaid (1989) Freeform Thur. 7 p.m. Modern Times (1936) TCM Thur. 5 p.m. Oliver! (1968) TCM Tues. 8:45 p.m. Rear Window (1954) Showtime Fri. 6:30 a.m. The Road Warrior (1981) BBC America Mon. 4 a.m. BBC America Fri. 10:15 a.m. Saving Private Ryan (1998) Showtime Wed. -

{Download PDF} the Informers Ebook Free Download
THE INFORMERS PDF, EPUB, EBOOK Bret Easton Ellis | 272 pages | 01 Apr 2011 | Pan MacMillan | 9780330536325 | English | London, United Kingdom The Informers PDF Book Strange that this was the top grossing film of all time and won 17 Oscars, as it's really quite off-putting. Release date. Over in the bad section of town lives Graham's doorman Jack, who's played by the late Brad Renfro in his final performance before his death from a heroin overdose in January From Wikipedia, the free encyclopedia. We are experiencing technical difficulties. The Informers. Cracking little tough-guy British crime picture. A potboiler - a bland helping of crime drama, with some well-known English character actors thrown in. Plot Keywords. Tim Fernando Consagra While it did lake some elements of a memorable film, it wasn't as good as a film like Crash for instance, But definitely worth my time and money. The acting is, on the whole, pretty bad. Did anyone try the Nigel Patrick? The Caryatids. Rarely seen now but worth tracking down. From metacritic. In Theaters. Summer of Scam 2: Criminally Insane. Alex Vetchinsky. Coming Soon. This movie is not as extreme as people believe it is. The Informers adaptation comes off as recycled, misplaced nostalgia at the hands of washed-up desperation trying to ring out his creative rag of the last drops to thirsty fans. The top-line talent, particularly Thornton and Rourke, do manage to hold our attention with idiosyncratic performances, but most of the others are a jumble of fair-haired, disaffected boys. Works by Bret Easton Ellis. -

Media's Positive and Negative Frames in Reporting Celebrity Deaths
Media’s Positive and Negative Frames in Reporting Celebrity Deaths From Illegal Drug Overdoses Versus Prescription Medication Overdoses By Michelle Wood Submitted to the William Allen White School of Journalism and Mass Communication and the Graduate Faculty of the University of Kansas in partial fulfillment of the requirements for the degree of Master of Science. ____________________________ Chairperson Mugur Geana, Assistant Professor _______________________________ John Broholm, Associate Professor _______________________________ Crystal Lumpkins, Assistant Professor Date Approved: November 14, 2011 ii The Thesis Committee for Michelle Wood certifies that this is the approved version of the following thesis: Media’s Positive and Negative Frames in Reporting Celebrity Deaths From Illegal Drug Overdoses Versus Prescription Medication Overdoses _______________________________ Mugur Geana, Assistant Professor Date Approved: November 14, 2011 iii Abstract This study compared the celebrity illegal drug overdose deaths of River Phoenix, Chris Farley, and Brad Renfro to the prescription drug overdose deaths of Anna Nicole Smith, Heath Ledger, and Brittany Murphy. This research used quantitative conceptual content analysis of news articles from The New York Times, The Los Angeles Times, People, and Entertainment Weekly. This research discovered whether media used in this study framed prescription drug deaths more positively than illegal drug deaths, which was proved to be true. It also determined media did not employ socially responsible frames when reporting on these deaths. The last question in this research discovered if the type of drug involved in the celebrities’ death influenced the way media portrayed the celebrities’ character. This research proved that, when reporting on negative drug overdoses, media framed celebrities’ perceptions negatively but found no evidence of positive celebrity perceptions when reporting about prescription drug overdoses. -

Performing Masculinity: the Star Persona of Tom Cruise Ruth O
Performing Masculinity: the Star Persona of Tom Cruise Ruth O’Donnell A thesis submitted in fulfilment of the requirements for the degree of Doctor of Philosophy Royal Holloway, University of London Department of Media Arts March 2012 1 Contents Acknowledgements p.6 Declaration p.7 Abstract p.8 Introduction: Masculinity and Performance in the Screen Persona of Tom Cruise p.9 Why a Study of Tom Cruise? p.9 Cruise‘s Star Persona p.10 Cruise and 1980s America p.11 A Psychodynamic Approach to Cruise p.17 Cruise Within Stardom p.19 Cruise Within Modern Hollywood p.26 Structure of the Thesis p.29 Section One: The Star Persona p.29 Section Two: Cruise‘s Performances of Masculinity p.31 Section Three: A Psychodynamic Reading of the Persona p.34 Conclusion p.38 Notes p.39 Chapter One: The Tom Cruise Persona p.40 Meanings of Persona p.40 Cruise‘s Persona p.44 The Early Persona of Tom Cruise p.46 Top Gun and Stardom p.52 Tom Cruise as Actor p.55 2 Extensions of the Persona p.58 Transgressing the Persona p.61 Eyes Wide Shut – a Shift in Persona p.65 Cruise, Oprah and Scientology p.71 Conclusion p.77 Notes p.78 Chapter Two: Male Bonding - Gender, Homoeroticism and the Performing Tom Cruise p.80 Bonds Between Men p.80 Performing Masculinity p.83 Figuring the Soldier p.85 The Other‘s Martial Masculinity p.91 Making an Exhibition of Oneself p.97 ‗Being the Best‘ – the Erotics of Male Competition p.106 ‗Passing‘ as Men p.112 Hysterical Performances p.119 Conclusion p.123 Notes p.124 Chapter Three: Male Bonding – The Racial Other p.126 Filmic Contexts