Investigating Foaming Solutions Generated by Naoh Extraction Of
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Spectral Clustering on Mercury Hollows: the Dominici Crater Case
Lunar and Planetary Science XLVIII (2017) 1329.pdf SPECTRAL CLUSTERING ON MERCURY HOLLOWS: THE DOMINICI CRATER CASE. A. Lucchetti1, M. Pajola2,3,1, G. Cremonese1, C. Carli4, G. A. Marzo5 and T. Roush3, 1INAF-Astronomical Observatory of Padova, Vicolo dell’Osservatorio 5, 35131 Padova, Italy ([email protected] ), 2Universities Space Research Association, NASA NPP Program (Supported by an appointment at NASA Ames Research Center: [email protected]), 3NASA Ames Research Center, Moffett Field, CA 94035, USA; 4INAF-IAPS Roma, Istituto di Astrofisica e Planetologia Spaziali di Roma, Via del Fosso del Cavaliere, 00133 Rome, Italy; 5ENEA Centro Ricerche Casaccia, 00123 Rome, Italy. Introduction: The Mercury Dual Imaging System [8], i.e. incidence angle of 30°, emission angle of 0° (MDIS, [1]) onboard NASA MESSENGER (MErcury and phase angle of 30°. On the photometrically cor- Surface, Space ENvironment, GEochemistry, and rected dataset we applied a statistical clustering over Ranging) spacecraft, provided the first global coverage the entire dataset based on a K-means partitioning al- of Mercury's surface with varying spatial resolution. gorithm [9]. It was developed and evaluated by [9-11] Early in the mission, high-resolution images showed and makes use of the Calinski and Harabasz criterion that specific areas exhibiting high reflectance and rela- [12] to find the intrinsically natural number of clusters, tive bluer in color were composed of shallow, irregular making the process unsupervised. A natural number of and rimless, flat-floored depressions with bright interi- ten clusters was identified within the crater and its ors and halos, often found on crater walls, rims, floors closest surroundings, see Fig. -

Declaration in Support of Plaintiffs
Case: 18-36082, 02/07/2019, ID: 11183380, DktEntry: 21-12, Page 1 of 80 Case No. 18-36082 IN THE UNITED STATES COURT OF APPEALS FOR THE NINTH CIRCUIT KELSEY CASCADIA ROSE JULIANA, et al., Plaintiffs-Appellees, v. UNITED STATES OF AMERICA, et al., Defendants-Appellants. On Interlocutory Appeal Pursuant to 28 U.S.C. § 1292(b) DECLARATION OF STEVEN W. RUNNING IN SUPPORT OF PLAINTIFFS’ URGENT MOTION UNDER CIRCUIT RULE 27-3(b) FOR PRELIMINARY INJUNCTION JULIA A. OLSON PHILIP L. GREGORY (OSB No. 062230, CSB No. 192642) (CSB No. 95217) Wild Earth Advocates Gregory Law Group 1216 Lincoln Street 1250 Godetia Drive Eugene, OR 97401 Redwood City, CA 94062 Tel: (415) 786-4825 Tel: (650) 278-2957 ANDREA K. RODGERS (OSB No. 041029) Law Offices of Andrea K. Rodgers 3026 NW Esplanade Seattle, WA 98117 Tel: (206) 696-2851 Attorneys for Plaintiffs-Appellees Case: 18-36082, 02/07/2019, ID: 11183380, DktEntry: 21-12, Page 2 of 80 I, Steven W. Running, hereby declare and if called upon would testify as follows: 1. In this Declaration, I offer my expert opinion about how excessive greenhouse gas (GHG) emissions, largely from the burning of fossil fuels, are causing climate change that is dangerously warming the surface of the Earth and causing devastating impacts to the Youth Plaintiffs in this case. Because there is a decades-long delay between the release of carbon dioxide (CO2) and the resultant warming of the climate, these Youth Plaintiffs have not yet experienced the full amount of warming that will occur from emissions already released. -

Thieves® Whitening Toothpaste
THIEVES® WHITENING TOOTHPASTE PRODUCT SUMMARY Start your morning on the bright side with the natural power of Thieves® Whitening Toothpaste. Pure and safe ingredients combine to whiten teeth, fight plaque, support healthy gums, and remove stains without damaging enamel. Great for the whole family, Young Living’s exclusive formula is both fluoride free and free of other harsh ingredients, offering you a superior clean you can be confident in. Grin from ear to ear knowing you made a fresh choice with Thieves Whitening Toothpaste! KEY INGREDIENTS Calcium hydroxyapatite, Silica, Perlite, Xylitol, Calcium bicarbonate, Thieves essential oil blend, Spearmint essential oil, Peppermint essential oil, Orange essential oil EXPERIENCE BENEFITS & FEATURES Smile bright with Thieves Whitening Toothpaste. • Whitens and brightens teeth Thieves fans will fall in love with this fresh take on Thieves-infused toothpaste. Fresh Peppermint, • Gently removes surface stains and cleans teeth cool Spearmint, and zesty Orange essential oils • Free from harmful peroxides combine with our spicy Thieves blend to leave your breath EO-fresh all day. Pure and safe whitening • Helps reduce and prevent tartar buildup agents leave you with long-lasting freshness and • Formulated without fluoride, SLS, parabens, a sparkling smile! phthalates, mineral oil, synthetic perfumes or dyes, toxic ingredients, or artificial colors, flavors, or preservatives PRODUCT BACKGROUND • Fights plaque and supports healthy gums Thieves Whitening Toothpaste is one more way Young Living is bringing the power of nature to your • Provides a long-lasting, smooth, fresh feel daily routine: a toothpaste gentle enough for every • Fluoride free and cruelty free day, formulated with essential oils and other natural • Formulated without GMOs ingredients. -

2019 Publication Year 2020-12-22T16:29:45Z Acceptance
Publication Year 2019 Acceptance in OA@INAF 2020-12-22T16:29:45Z Title Global Spectral Properties and Lithology of Mercury: The Example of the Shakespeare (H-03) Quadrangle Authors BOTT, NICOLAS; Doressoundiram, Alain; ZAMBON, Francesca; CARLI, CRISTIAN; GUZZETTA, Laura Giovanna; et al. DOI 10.1029/2019JE005932 Handle http://hdl.handle.net/20.500.12386/29116 Journal JOURNAL OF GEOPHYSICAL RESEARCH (PLANETS) Number 124 RESEARCH ARTICLE Global Spectral Properties and Lithology of Mercury: The 10.1029/2019JE005932 Example of the Shakespeare (H-03) Quadrangle Key Points: • We used the MDIS-WAC data to N. Bott1 , A. Doressoundiram1, F. Zambon2 , C. Carli2 , L. Guzzetta2 , D. Perna3 , produce an eight-color mosaic of the and F. Capaccioni2 Shakespeare quadrangle • We identified spectral units from the 1LESIA-Observatoire de Paris-CNRS-Sorbonne Université-Université Paris-Diderot, Meudon, France, 2Istituto di maps of Shakespeare 3 • We selected two regions of high Astrofisica e Planetologia Spaziali-INAF, Rome, Italy, Osservatorio Astronomico di Roma-INAF, Monte Porzio interest as potential targets for the Catone, Italy BepiColombo mission Abstract The MErcury Surface, Space ENvironment, GEochemistry and Ranging mission showed the Correspondence to: N. Bott, surface of Mercury with an accuracy never reached before. The morphological and spectral analyses [email protected] performed thanks to the data collected between 2008 and 2015 revealed that the Mercurian surface differs from the surface of the Moon, although they look visually very similar. The surface of Mercury is Citation: characterized by a high morphological and spectral variability, suggesting that its stratigraphy is also Bott, N., Doressoundiram, A., heterogeneous. Here, we focused on the Shakespeare (H-03) quadrangle, which is located in the northern Zambon, F., Carli, C., Guzzetta, L., hemisphere of Mercury. -

2D Mercury Crater Wordsearch V2
3/24/2019 Word Search Generator :: Create your own printable word find worksheets @ A to Z Teacher Stuff MAKE YOUR OWN WORKSHEETS ONLINE @ WWW.ATOZTEACHERSTUFF.COM NAME:_______________________________ DATE:_____________ Craters on Mercury SICINIMODFIQPVMRQSLJ BEETHOVEN MICHELANGELO BLTVPTSDUOMRCIPDRAEN BYRON RAPHAEL YAPVWYPXSEHAUEHSEVDI CUNNINGHAM SAVAGE RRZAYRKFJROGNIGSNAIA DAMER SHAKESPEARE ORTNPIVOCDTJNRRSKGSW DOMINICI SVEINSDOTTIR NOMGETIKLKEUIAAGLEYT DRISCOLL TOLSTOI PCLOLTVLOEPSNDPNUMQK ELLINGTON VANGOGH YHEGLOAAEIGEGAHQAPRR FAULKNER VIEIRADASILVA NANHIDLNTNNNHSAOFVLA HEMINGWAY VIVALDI VDGYNSDGGMNGAIEDMRAM HOLST GALQGNIEBIMOMLLCNEZG HOMER VMESTIWWKWCANVEKLVRU IMHOTEP ZELTOEPSBOAWMAUHKCIS IZQUIERDO JRQGNVMODREIUQZICDTH JOPLIN SHAKESPEARETOLSTOIOX KIPLING BBCZWAQSZRSLPKOJHLMA LANGE SFRLLOCSIRDIYGSSSTQT LARROCHA FKUIDTISIYYFAIITRODE LENGLE NILPOJHEMINGWAYEGXLM LENNON BEETHOVENRYSKIPLINGV MARKTWAIN 1/2 Mercury Craters: Famous Writers, Artists, and Composers: Location and Sizes Beethoven: Ludwig van Beethoven (1770−1827). German composer and pianist. 20.9°S, 124.2°W; Diameter = 630 km. Byron: Lord Byron (George Byron) (1788−1824). British poet and politician. 8.4°S, 33°W; Diameter = 106.6 km. Cunningham: Imogen Cunningham (1883−1976). American photographer. 30.4°N, 157.1°E; Diameter = 37 km. Damer: Anne Seymour Damer (1748−1828). English sculptor. 36.4°N, 115.8°W; Diameter = 60 km. Dominici: Maria de Dominici (1645−1703). Maltese painter, sculptor, and Carmelite nun. 1.3°N, 36.5°W; Diameter = 20 km. Driscoll: Clara Driscoll (1861−1944). American glass designer. 30.6°N, 33.6°W; Diameter = 30 km. Ellington: Edward Kennedy “Duke” Ellington (1899−1974). American composer, pianist, and jazz orchestra leader. 12.9°S, 26.1°E; Diameter = 216 km. Faulkner: William Faulkner (1897−1962). American writer and Nobel Prize laureate. 8.1°N, 77.0°E; Diameter = 168 km. Hemingway: Ernest Hemingway (1899−1961). American journalist, novelist, and short-story writer. 17.4°N, 3.1°W; Diameter = 126 km. -

MSDS for Anti-Foaming Agent
MATERIAL SAFETY DATA SHEET for Anti-Foaming Agent Revision: 1.0 1/19/2005 Page 1 of 8 1. PRODUCT AND COMPANY IDENTIFICATION Product Name: Anti-Foaming Agent Chemical Name: Polydimethylsiloxane emulsion Supplier: The Real Milk Paint Co. 11 W. Pumping Station Rd. Quakertown, PA 18951 Contact Numbers: CHEM-TELL (24 Hours) 800-255-0573 Customer Service 800-339-9748 2. COMPOSITION/INFORMATION ON INGREDIENTS COMPOENT CAS3 CONCENTRATION Water 7732-18-5 >50.0% Proprietary additives Trade Secret <30.0% Silica filled polydimethylsiloxane Trade Secret <15.0% Note(s): See Section 15 for chemicals appearing on Federal or State Right-TO-Know lists. 3. HAZARDOUS IDENTIFICATION EMERGENCY OVERVIEW NORMAL PRECAUTIONS COMMON TO SAFE MANUFACTURING PRACTICE SHOULD BE FOLLOWED IN HANDLING AND STORAGE. 4. FIRST AID MEASURES Swallowing No emergency care anticipated Skin Wash skin with soap and water MATERIAL SAFETY DATA SHEET for Anti-Foaming Agent Revision: 1.0 1/19/2005 Page 2 of 8 Inhalation No emergency care anticipated. Eye contact Flush eyes thoroughly with water for several minutes Notes to physicians Toxicology studies have shown this material to be of very low acute toxicity. There is no specific antidote. Treatment of overexposure should be directed at the control of symptoms and the clinical condition of the patient. 5. FIRE FIGHTING MEASURES Flammable limits Lower limits Not available Upper limits Not available Special fire fighting procedures None. Special protective equipment for firefighters Use self-contained breathing apparatus when fighting fires in enclosed areas. Extinguishing media Suitable: Non-flammable (aqueous) After water evaporates, the remaining material will burn. Large fires: -Alcohol-type foam or universal type foam Small fires: -CO2 -dry chemical Unsuitable: None. -

Injection Molding Foam Guidelines
SAFOAM USER’S GUIDE This ‘SAFOAM User’s guide’ was prepared as a service to the customers using Reedy International Corporation’s SAFOAM family of endothermic foaming agents. Reproduction or distribution of this brochure, in whole or in part, is prohibited without the permission of Reedy International Corporation. TABLE OF CONTENTS 1. Introduction ........................................................................................................1 SAFOAM - Its Use in Thermoplastics ........................................................2 2. Structural Plastics and Polymer Selection .........................................................3 Properties of Amorphous Polymers .............................................................3 Properties of Crystalline Polymers ..............................................................4 Properties of Thermoplastic Elastomers ......................................................4 3. Foaming Agent Selection ...................................................................................6 Physical Foaming Agents ............................................................................7 Chemical Foaming Agents ...........................................................................7 Exothermic Chemical Foaming Agents Azodicarbonamide .................................................................................8 Endothermic Chemical Foaming Agents Sodium Borohydride ..............................................................................9 Traditional Acid/Carbonate Systems -
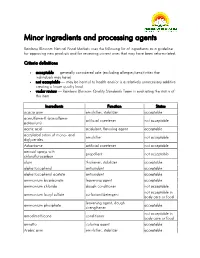
Minor Ingredients and Processing Agents
Minor ingredients and processing agents Rainbow Blossom Natural Food Markets uses the following list of ingredients as a guideline for approving new products and for reviewing current ones that may have been reformulated. Criteria definitions acceptable — generally considered safe (excluding allergies/sensitivities that individuals may have) not acceptable — may be harmul to health and/or is a relatively unnecessary additive creating a lower quality food under review — Rainbow Blossom Quality Standards Team is evaluating the status of this item Ingredients Function Status acacia gum emulsifier, stabilizer acceptable acesulfame-K (acesulfame- artificial sweetener not acceptable potassium) acetic acid acidulant, flavoring agent acceptable acetylated esters of mono- and emulsifier not acceptable diglycerides Advantame artificial sweetener not acceptable aerosol sprays with propellant not acceptable chloroflurocarbon algin thickener, stabilizer acceptable alpha tocopherol antioxidant acceptable alpha tocopherol acetate antioxidant acceptable ammonium bicarbonate leavening agent acceptable ammonium chloride dough conditioner not acceptable not acceptable in ammonium lauryl sulfate surfactant/detergent body care or food leavening agent, dough ammonium phosphate acceptable strengthener not acceptable in amodimethicone conditioner body care or food annatto coloring agent acceptable Arabic gum emulsifier, stabilizer acceptable Ingredients Function Status artificial colors (red #3, red # 40, yellow #5, yellow #6, blue #1, blue coloring agent -

1 the Lifecycle of Hollows on Mercury
The Lifecycle of Hollows on Mercury: An Evaluation of Candidate Volatile Phases and a Novel Model of Formation. 1 1 2 3 M. S. Phillips , J. E. Moersch , C. E. Viviano , J. P. Emery 1Department of Earth and Planetary Sciences, University of Tennessee, Knoxville 2Planetary Exploration Group, Johns Hopkins University Applied Physics Laboratory 3Department of Astronomy and Planetary Sciences, Northern Arizona University Corresponding author: Michael Phillips ([email protected]) Keywords: Mercury, hollows, thermal model, fumarole. Abstract On Mercury, high-reflectance, flat-floored depressions called hollows are observed nearly globally within low-reflectance material, one of Mercury’s major color units. Hollows are thought to be young, or even currently active, features that form via sublimation, or a “sublimation-like” process. The apparent abundance of sulfides within LRM combined with spectral detections of sulfides associated with hollows suggests that sulfides may be the phase responsible for hollow formation. Despite the association of sulfides with hollows, it is still not clear whether sulfides are the hollow-forming phase. To better understand which phase(s) might be responsible for hollow formation, we calculated sublimation rates for 57 candidate hollow-forming volatile phases from the surface of Mercury and as a function of depth beneath regolith lag deposits of various thicknesses. We found that stearic acid (C18H36O2), fullerenes (C60, C70), and elemental sulfur (S) have the appropriate thermophysical properties to explain hollow formation. Stearic acid and fullerenes are implausible hollow-forming phases because they are unlikely to have been delivered to or generated on Mercury in high enough volume to account for hollows. -

Overview and Current Status of Remote Sensing Applications Based on Unmanned Aerial Vehicles (Uavs)
Overview and Current Status of Remote Sensing Applications Based on Unmanned Aerial Vehicles (UAVs) Gonzalo Pajares Abstract Remotely Piloted Aircraft (RPA) is presently in continuous battery or energy system’s capabilities. There are vehicles with development at a rapid pace. Unmanned Aerial Vehicles the ability to fly at medium and high altitudes with flight dura- (UAVs) or more extensively Unmanned Aerial Systems (UAS) tions ranging from minutes to hours, i.e., from five minutes are platforms considered under the RPAs paradigm. Simulta- to 30 hours. The horizontal range of the different platforms neously, the development of sensors and instruments to be is also limited by the power of the communications system, installed onboard such platforms is growing exponentially. which should ensure contact with a ground station, again These two factors together have led to the increasing use of ranging from meters to kilometers. Communications using sat- these platforms and sensors for remote sensing applications ellite input can also be used, expanding the operational range. with new potential. Thus, the overall goal of this paper is There are several different categorizations for unmanned aerial to provide a panoramic overview about the current status platforms depending on the criterion applied (Nonami et al., of remote sensing applications based on unmanned aerial 2010). Perhaps the most extensive and current classifications platforms equipped with a set of specific sensors and instru- can be found in Blyenburgh (2014) with annual revisions. ments. First, some examples of typical platforms used in An auto platform or remotely controlled platform through remote sensing are provided. Second, a description of sensors a remote station together with a communication system, and technologies is explored which are onboard instruments including the corresponding protocol, constitutes what is specifically intended to capture data for remote sensing ap- known an Unmanned Aircraft System (UAS) (Gertler, 2012). -

The Waning Sword E Conversion Imagery and Celestial Myth in Beowulf DWARD the Waning Sword Conversion Imagery and EDWARD PETTIT P
The Waning Sword E Conversion Imagery and Celestial Myth in Beowulf DWARD The Waning Sword Conversion Imagery and EDWARD PETTIT P The image of a giant sword mel� ng stands at the structural and thema� c heart of the Old ETTIT Celestial Myth in Beowulf English heroic poem Beowulf. This me� culously researched book inves� gates the nature and signifi cance of this golden-hilted weapon and its likely rela� ves within Beowulf and beyond, drawing on the fi elds of Old English and Old Norse language and literature, liturgy, archaeology, astronomy, folklore and compara� ve mythology. In Part I, Pe� t explores the complex of connota� ons surrounding this image (from icicles to candles and crosses) by examining a range of medieval sources, and argues that the giant sword may func� on as a visual mo� f in which pre-Chris� an Germanic concepts and prominent Chris� an symbols coalesce. In Part II, Pe� t inves� gates the broader Germanic background to this image, especially in rela� on to the god Ing/Yngvi-Freyr, and explores the capacity of myths to recur and endure across � me. Drawing on an eclec� c range of narra� ve and linguis� c evidence from Northern European texts, and on archaeological discoveries, Pe� t suggests that the T image of the giant sword, and the characters and events associated with it, may refl ect HE an elemental struggle between the sun and the moon, ar� culated through an underlying W myth about the the� and repossession of sunlight. ANING The Waning Sword: Conversion Imagery and Celesti al Myth in Beowulf is a welcome contribu� on to the overlapping fi elds of Beowulf-scholarship, Old Norse-Icelandic literature and Germanic philology. -

Visibility Rule and BART for Regional Haze, Final
Regulatory Impact Analysis for the Final Clean Air Visibility Rule or the Guidelines for Best Available Retrofit Technology (BART) Determinations Under the Regional Haze Regulations EPA-452/R-05-004 June 2005 Regulatory Impact Analysis for the Final Clean Air Visibility Rule or the Guidelines for Best Available Retrofit Technology (BART) Determinations Under the Regional Haze Regulations U.S. Environmental Protection Agency Office of Air and Radiation Air Quality Strategies and Standards Division, Emission, Monitoring, and Analysis Division and Clean Air Markets Division CONTENTS Section Page 1. EXECUTIVE SUMMARY ........................................ 1-1 1.1 Background .............................................. 1-1 1.2 Results .................................................. 1-2 1.2.1 Health Benefits ...................................... 1-4 1.2.2 Welfare Benefits .................................... 1-7 1.2.3 Uncertainty in the Benefits Estimates .................... 1-7 1.3 Not All Benefits Quantified .................................. 1-8 1.4 Costs and Economic Impacts ................................ 1-10 1.5 Limitations .............................................. 1-11 1.6 References .............................................. 1-12 2. INTRODUCTION AND BACKGROUND ............................ 2-1 2.1 Background .............................................. 2-1 2.2 Regulated Source Categories ................................. 2-3 2.3 Control Scenarios.......................................... 2-4 2.3.1 Electric Generating