Oxidation of Cycloalkanes Catalysed by N-Hydroxyimides in Supercritical Carbon Dioxide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
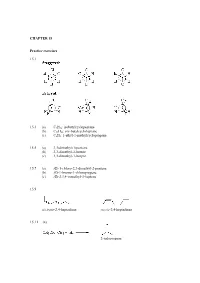
CHAPTER 15 Practice Exercises 15.1 15.3 (A) C9H18
CHAPTER 15 Practice exercises 15.1 15.3 (a) C9H18: isobutylcyclopentane (b) C11H22: sec-butylcycloheptane (c) C6H2: 1-ethyl-1-methylcyclopropane 15.5 (a) 3,3-dimethyl-1-pentene (b) 2,3-dimethyl-2-butene (c) 3,3-dimethyl-1-butyne 15.7 (a) (E)-1-chloro-2,3-dimethyl-2-pentene (b) (Z)-1-bromo-1-chloropropene (c) (E)-2,3,4-trimethyl-3-heptene 15.9 cis,trans-2,4-heptadiene cis,cis-2,4-heptadiene 15.11 (a) 2-iodopropane (b) 1-iodo-1-methylcyclohexane 15.13 CH3 CH3 + HI I Step 1: Protonation of the alkene to give the most stable 3˚ carbocation intermediate: slow step + CH3 HI CH3 I rate-determining step Step 2: Nucleophilic attack of the iodide anion on the 3˚ carbocation intermediate to give the product: CH + 3 CH3 I I 15.15 H2O CH3 CH3 + H3O OH Step 1: Protonation of the alkene to give the most stable 3˚ carbocation intermediate: slow step H + + CH3 HOH CH3 O H rate-determining H step Step 2: Nucleophilic attack of the water on the 3˚ carbocation intermediate to give the protonated alcohol: H H O+ H + CH3 O H CH3 Step 3: The protonated alcohol loses a proton to form the product: H + O H H CH3 + O + H3O CH3 H OH 15.17 (a) 2-phenyl-2-propanol (b) (E)-3,4-diphenyl-3-hexene (c) 3-methylbenzoic acid or m-methylbenzoic acid Review questions 15.1 A hydrocarbon is a compound composed only of hydrogen and carbon atoms. 15.3 In saturated hydrocarbons, each carbon is bonded to four other atoms, either hydrogen or carbon atoms. -

In This Handout, All of Our Functional Groups Are Presented As Condensed Line Formulas, 2D and 3D Formulas and with Nomenclature Prefixes and Suffixes (If Present)
In this handout, all of our functional groups are presented as condensed line formulas, 2D and 3D formulas and with nomenclature prefixes and suffixes (if present). Organic names are built on a foundation of alkanes, alkenes and alkynes. Those examples are presented first and you need to know those rules. The strategies can be found in Chapter 4 of our textbook (alkanes: pages 93-98, cycloalkanes 102-104, alkenes: pages 104-110, alkynes: pages 112-113 and combinations of all of them 113-115). After introducing examples of alkanes, alkenes, alkynes and combinations of them, the functional groups are presented in order of priority. A few nomenclature examples are provided for each of the functional groups. Examples of the various functional groups are presented on pages 115-135 in the textbook. Two overview pages are on pages 136-137. Some functional groups have a suffix name when they are the highest priority functional group and a prefix name when they are not the highest priority group, and these are added to the skeletal names with identifying numbers and stereochemistry terms (E and Z for alkenes, R and S for chiral centers and cis and trans for rings). Several low priority functional groups only have a prefix name. A few additional special patterns are shown on pages 98-102. The only way to learn this topic is practice (over and over). The best practice approach is to actually write out the names (on an extra piece of paper or on a white board, and then do it again). The same functional groups are used throughout the entire course. -

THE EFFECTS of SEVERAL PAPER CHARACTERISTICS and APPLICATION METHODS on the SUBLIMATION RATE of CYCLODODECANE by Kelli A. Piot
THE EFFECTS OF SEVERAL PAPER CHARACTERISTICS AND APPLICATION METHODS ON THE SUBLIMATION RATE OF CYCLODODECANE by Kelli A. Piotrowski A thesis submitted to the Department of Art In conformity with the requirements for the degree of Master of Art Conservation Queen’s University Kingston, Ontario, Canada (September, 2013) Copyright © Kelli Piotrowski, 2013 ABSTRACT Cyclododecane (CDD) is a waxy, solid cyclic hydrocarbon (C12H24) that sublimes at room temperature and possesses strong hydrophobicity. In paper conservation CDD is used principally as a temporary fixative of water-soluble media during aqueous treatments. Hydrophobicity, ease of reversibility, low toxicity, and absence of residues are reasons often cited for its use over alternative materials although the latter two claims continue to be debated in the literature. The sublimation rate has important implications for treatment planning as well as health and safety considerations given the dearth of reliable information on its toxicity and exposure limits. This study examined how the rate of sublimation is affected by fiber type, sizing, and surface finish as well as delivery in the molten phase and as a saturated solution in low boiling petroleum ether. The effect of warming the paper prior to application was also evaluated. Sublimation was monitored using gravimetric analysis after which samples were tested for residues with gas chromatography-flame ionization detection (GC-FID) to confirm complete sublimation. Water absorbency tests were conducted to determine whether this property is fully reestablished. Results suggested that the sublimation rate of CDD is affected minimally by all of the paper characteristics and application methods examined in this study. The main factors influencing the rate appear to be the thickness and mass of the CDD over a given surface area as well as temperature and ventilation. -
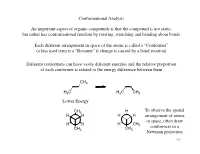
Conformational Analysis an Important Aspect of Organic Compounds Is That
Conformational Analysis An important aspect of organic compounds is that the compound is not static, but rather has conformational freedom by rotating, stretching and bending about bonds Each different arrangement in space of the atoms is called a “Conformer” (a less used term is a “Rotamer” if change is caused by a bond rotation) Different conformers can have vastly different energies and the relative proportion of each conformer is related to the energy difference between them CH3 H3C H3C CH3 Lower Energy CH3 H To observe the spatial H H H H arrangement of atoms in space, often draw H H H CH 3 conformers in a CH CH3 3 Newman projection 196 Conformational Analysis Conformers will be of different energy due to strain Sources of strain are generally categorized in one of three types: 1) Torsional strain Torsional strain is due to interactions as groups change relative position with a change in torsional bond angle Consider rotation of ethane H H Eclipsed Eclipsed is higher in H H H conformation energy than staggered H due to increased ~3 kcal/mol torsional strain y g r e n E Further rotation will H H convert eclipsed Staggered H H H H conformation back to conformation H H H H staggered H H -60˚ 0˚ 60˚ 120˚ 180˚ 240˚ 197 torsional angle Conformational Analysis 2) van der Waals strain Another source of strain is when groups are placed in positions closer than the sum of their van der Waals radii Consider rotation of butane totally eclipsed eclipsed CH H3C 3 CH H 3 H H H “totally eclipsed” CH3 H H H conformation (which has H largest groups eclipsing each other) is higher in energy than y other eclipsed conformations g r e n E “gauche” conformation is CH3 CH3 H CH3 higher in energy than anti H H (both are “staggered” H H H H H conformations) CH3 anti gauche -60˚ 0˚ 60˚ 120˚ 180˚ 240˚ 198 torsional angle Conformational Analysis The difference in energy thus affects the amount of each conformer present n-Butane CH3 H H H H Majority of CH3 compound is in the anti conformation, hardly any in CH3 H CH eclipsed 3 conformation H H Mole Fraction H Torsional Angle W.L. -

Reactions of OH Radicals with C6АC10 Cycloalkanes in The
ARTICLE pubs.acs.org/JPCA Reactions of OH Radicals with C6ÀC10 Cycloalkanes in the Presence of NO: Isomerization of C7ÀC10 Cycloalkoxy Radicals † † ‡ † ‡ § Sara M. Aschmann, Janet Arey,*, , and Roger Atkinson*, , , † ‡ § Air Pollution Research Center, Department of Environmental Sciences, and Department of Chemistry, University of California, Riverside, California 92521, United States ABSTRACT: Rate constants have been measured for the reactions of OH À ( radicals with a series of C6 C10 cycloalkanes and cycloketones at 298 2K, by a relative rate technique. The measured rate constants (in units of À À À 10 12 cm3 molecule 1 s 1) were cycloheptane, 11.0 ( 0.4; cyclooctane, 13.5 ( 0.4; cyclodecane, 15.9 ( 0.5; cyclohexanone, 5.35 ( 0.10; cyclo- heptanone, 9.57 ( 0.41; cyclooctanone, 15.4 ( 0.7; and cyclodecanone, 20.4 ( 0.8, where the indicated errors are two least-squares standard deviations and do not include uncertainties in the rate constant for the reference compound n-octane. Formation yields of cycloheptanone from cycloheptane (4.2 ( 0.4%), cyclooctanone from cyclooctane (0.85 ( 0.2%), and cyclodecanone from cyclodecane (4.9 ( 0.5%) were also determined by gas chromatography, where the molar yields are in parentheses. Analyses of products by direct air sampling atmospheric pressure ionization mass spectrometry and by combined gas chromatographyÀmass spectrometry showed, in addition to the cycloketones, the presence of cycloalkyl nitrates, cyclic hydroxyketones, hydroxydicarbonyls, hydroxycarbonyl nitrates, and products attributed to carbonyl nitrates and/or cyclic hydroxynitrates. The observed formation of cyclic hydroxyketones from the cycloheptane, cyclooctane and cyclodecane reactions, with estimated molar yields of 46%, 28%, and 15%, respectively, indicates the occurrence of cycloalkoxy radical isomerization. -

Cyclohexene Oxide 286-20-4 NTP NOMINATION HISTORY and REVIEW
Cyclohexene oxide 286-20-4 NTP NOMINATION HISTORY AND REVIEW A. Nomination History 1. Source: National cancer Institute 2. Recommendations: -Mechanistic carcinogencity studies -Synergism studies or two-stage studies - Test with benzene or other chemicals deemed appropriate by NTP 3. Rationale/Remarks: -High annual production volume -Potential for human exposure based on its use as a research and industrial chemical -Environmental contaminant -Representative cycloalkene monoepoxide; suspicion of carcinogenicity as a member of the epoxides· chemical class 4. Priority: High 5. Date of Nomination: 4/93 B. Interagency Committee for Chemical Evaluation and Coordination Review \ 1. Date of Review: 2. Recommendations: 3. Priority: 4. NTP Chemical Selection Principles: 5. Rationale/Remarks: "' 286-20-4 Cyclohexene oxide SUMMARY OF DATA FOR CHEMICAL SELECTION CHEMICAL IDENTIFICATION CAS Registry Name: 286-20-4 Chemical Abstracts Name: 7-oxabicyclo[ 4.1.0]heptane(9CI,8CI) Synonyms and Trade Names: Cyclohexene oxide; cyclohexene epoxide; 1,2 epoxycyclohexane; cyclohexane oxide tetramethyleneoxirane; CO; CHO Structure. Molecular Formula and Molecular Weight Mol. wt.: 98.15 Chemical and Physical Properties Description: Colorless liquid with strong (pungent, offensive) odor (Sax & Lewis, 1987;Uniroyal, 1990) Boiling Point 129-131°C; 266°F (Sax & Lewis, 1987; Uniroyal, 1990; Weast, 1989) Melting Point <-10°C (Weast, 1989) Solubilitv: Very soluble in benzene; soluble in alcohol, ether, acetone, chloroform; low water solubility (-0.5 g/L) (Sax & Lewis, 1987; BASF, 1990; Weast, 1989) DensitY: 0.967 g/cc@ 25°/4°C (Sax & Lewis, 1987; Uniroyal, 1990) Prepared for NCI by Technical Resources, Inc. under Contract No. N01-CP-56019 (9/91; rev. 2/93) 6'( II 286-20-4 Cyclohexene oxide Vapor Pressure: 12 mbar (BASF, 1990) Flash Point 27oC; 81 oF (Sax & Lewis, 1989; Uniroyal, 1990.) Stabiiitv: Stable at ambient temperatures and pressures. -

A Survey of Vapors in the Headspaces of Single-Shell Waste Tanks
PNNL-13366, Rev. 1 A Survey of Vapors in the Headspaces of Single-Shell Waste Tanks L. M. Stock J. L. Huckaby July 2004 Prepared for the U.S. Department of Energy under Contract DE-AC06-76RL01830 DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor Battelle Memorial Institute, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof, or Battelle Memorial Institute. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. PACIFIC NORTHWEST NATIONAL LABORATORY operated by BATTELLE for the UNITED STATES DEPARTMENT OF ENERGY under Contract DE-AC06-76RL01830 This document was printed on recycled paper. (8/00) PNNL-13366, Rev. 1 A Survey of Vapors in the Headspaces of Single-Shell Waste Tanks L. M. Stock J. L. Huckaby July 2004 Prepared for the U.S. Department of Energy under Contract DE-AC06-76RL01830 Pacific Northwest National Laboratory Richland, Washington 99352 Summary This report summarizes data on the organic vapors in the single-shell, high-level radioactive waste tanks at the Hanford Site. -

Table of Thermal Properties of Linear Macromolecules and Related Small Molecules—The ATHAS Data Bank A
__________________________________________________________________Appendix 1 777 Table of Thermal Properties of Linear Macromolecules and Related Small Molecules—The ATHAS Data Bank a 1. Poly(alkene)s............................................ 780 2. Poly(vinyl)s and Related Polymers........................... 781 3. Aliphatic Poly(oxide)s .................................... 783 4. Poly(acrylate)s and (methacrylate)s .......................... 784 5. Aliphatic Poly(ester)s..................................... 785 6. Poly(itaconate)s.......................................... 786 7. Aliphatic Poly(amide)s.................................... 787 8. Poly(amino Acid)s ....................................... 787 9. Phenylene-containing Polymers ............................. 789 10. Poly(silylene)s........................................... 792 11. Poly(siloxane)s.......................................... 792 12. Aliphatic and Aromatic Poly(sulfone)s........................ 793 13. Inorganic Polymers....................................... 793 14. Paraffins and Perfluoroparaffins............................. 793 15. Tetraalkylammonium Salts................................. 797 16. Other Small Molecules.................................... 798 17. References.............................................. 799 In the table the following symbols are used: The glass transition temperature is listed as Tg; Cp is the change of the heat capacity at Tg for the fully amorphous sample; Tm is the equilibrum melting temperature; Hf, the heat of fusion for -

7.2 Conformations of Cyclohexane 269
07_BRCLoudon_pgs5-1.qxd 12/8/08 12:13 PM Page 269 7.2 CONFORMATIONS OF CYCLOHEXANE 269 TABLE 7.1 Heats of Formation per CH2 for Some Cycloalkanes L L (n number of carbon atoms) \ DH°f Ün DH°f Ün 1 1 1 1 n Compound kJ mol_ kcal mol_ n Compound kJ mol_ kcal mol_ 3cyclopropane 17.8 4.25 9 cyclononane 14.8 3.5 + + - - 4cyclobutane 7.1 1.7 10 cyclodecane 15.4 3.7 + + - - 5cyclopentane 15.7 3.7 11 cycloundecane 16.3 3.9 - - - - 6cyclohexane 20.55 4.95 12 cyclododecane 19.2 4.6 - - - - 7cycloheptane 16.9 4.0 13 cyclotridecane 18.95 4.5 - - - - 8cyclooctane 15.55 3.7 14 cyclotetradecane 17.1 4.1 - - - - 1 1 exactly the same contribution to its heat of formation ( 20.7 kJ mol_ or 4.95 kcal mol_ ). This means that cyclohexane has the same stability as a- typical unbranched- alkane. Cyclohexane is the most widely occurring ring in compounds of natural origin. Its preva- lence, undoubtedly a consequence of its stability, makes it the most important of the cy- cloalkanes. The stability of cyclohexane is due to its conformation, which is the subject of Section 7.2. The stabilities and conformations of other cycloalkanes are discussed in Sec. 7.5. 7.2 CONFORMATIONS OF CYCLOHEXANE A. The Chair Conformation The stability data in Table 7.1 require that the bond angles in cyclohexane must be essentially the same as the bond angles in an alkane—very close to the ideal 109.5 tetrahedral angle. -

Seven Conformations of the Macrocycle Cyclododecanone Unveiled by Microwave Spectroscopy
molecules Article Seven Conformations of the Macrocycle Cyclododecanone Unveiled by Microwave Spectroscopy Ecaterina Burevschi and M. Eugenia Sanz * Department of Chemistry, King’s College London, London SE1 1DB, UK; [email protected] * Correspondence: [email protected] Abstract: The physicochemical properties and reactivity of macrocycles are critically shaped by their conformations. In this work, we have identified seven conformations of the macrocyclic ketone cyclododecanone using chirped-pulse Fourier transform microwave spectroscopy in combination with ab initio and density functional theory calculations. Cyclododecanone is strongly biased towards adopting a square configuration of the heavy atom framework featuring three C–C bonds per side. The substitution and effective structures of this conformation have been determined through the observation of its 13C isotopologues. The minimisation of transannular interactions and, to a lesser extent, HCCH eclipsed configurations drive conformational preferences. Our results contribute to a better understanding of the intrinsic forces mediating structural choices in macrocycles. Keywords: conformational flexibility; rotational spectroscopy; large ring; cycloketone; intramolecular interactions 1. Introduction Citation: Burevschi, E.; Sanz, M.E. Macrocycles are molecules containing twelve-membered or larger rings, used in drug Seven Conformations of the discovery [1], catalysis and materials [2]. Their stability, reactivity and physicochemi- Macrocycle Cyclododecanone cal characteristics are intimately related to their structural properties, specifically to the Unveiled by Microwave Spectroscopy. conformations they adopt and their intramolecular forces. Conformational analysis of Molecules 2021, 26, 5162. https:// macrocycles is crucial to characterise their ability to interact with other molecules, predict doi.org/10.3390/molecules26175162 possible changes in their configurations and design modified macrocycles with pre-selected conformations. -

United States Patent 19 11 Patent Number: 5,892,123 Anderson Et Al
USOO5892.123A United States Patent 19 11 Patent Number: 5,892,123 Anderson et al. (45) Date of Patent: Apr. 6, 1999 54 PROCESS FOR REPRODUCING A MIXTURE 56) References Cited CONTAINING CYCLODODECANONE AND CYCLODODECANOL U.S. PATENT DOCUMENTS 75 Inventors: Howard Wayne Anderson, Hockessin; 4,465,861 8/1984 Hermollin - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 568/342 James Bernard Sieja, Newark, both of 4,568,769 2/1986 Yashima - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 568/342 Del. FOREIGN PATENT DOCUMENTS 73 Assignee: E. I. du Pont de Nemours and 1032390 6/1966 United Kingdom. Company, Wilmington, Del. 21 Appl. No.: 17,784 Primary Examiner Shailendra Kumar ASSistant Examiner Sreeni Padmanabhan 22 Filed: Feb. 3, 1998 57 ABSTRACT 51 Int. Cl. ............................ C07C 45/53; CO7C 35/08 52 U.S. Cl. .......................... 568/361; 568/342; 568/344; A method is disclosed for catalytically decomposing 568/821 cyclododecyl hydroperoxide into cyclododecanone and 58 Field of Search ..................................... 568/342,835, cyclododecanol using a chromium catalyst. 568/798, 385,578, 361, 821, 375,832, 344 7 Claims, No Drawings 5,892,123 1 2 PROCESS FOR REPRODUCING AMIXTURE cyclododecane, is decomposed to cyclododecanone (K) and CONTAINING CYCLODODECANONE AND cyclododecanol (A) in the presence of a chromium catalyst. CYCLODODECANOL Cyclododecyl hydroperoxide may be obtained by oxida tion of cyclododecane. Air oxidation is the preferred BACKGROUND OF THE INVENTION method. This may be performed by bubbling air through Cyclododecanone (K) and cyclododecanol (A) are inter cyclododecane at a temperature of about 145 C. to about mediates in the production of nylon-12 and 1,12 180° C. Normally conversion is about 10%. The resulting dodecanedioic acid (DDDA). -

I a Database of Produced Water Constituents with Ranking Of
A Database of Produced Water Constituents with Ranking of Human Health Risk By Sydney M. Joffre B.S. Chemical Engineering, University of Colorado, 2018 B.S. Environmental Engineering, University of Colorado, 2019 A thesis submitted to the Faculty of the Graduate School of the University of Colorado in partial fulfillment of the requirement for the degree Master of Science Department of Civil, Environmental, and Architectural Engineering 2020 Committee Members: Cloelle Danforth Karl Linden James Rosenblum Joseph Ryan i Abstract: Sydney M. Joffre (Master of Science, Civil, Environmental, and Architectural Engineering) A Database of Produced Water Constituents with Ranking of Human Health Risk Thesis Directed by Professor Joseph N. Ryan Produced water is the largest waste stream of upstream oil and gas production in terms of volume. This study aims to address the implications of produced water reuse applications and inadvertent releases. We created a database of compounds identified in produced water from onshore oil and gas operations in North America and developed a prioritization scheme for those chemicals based on potential risk to human health. Through a comprehensive literature review, we found 179 studies that met our inclusion criteria. In total, there were 1,337 chemicals with a Chemical Abstract Service (CAS) number and 41 general water quality parameters (e.g., total dissolved solids, alkalinity) in produced water reported by the studies. We used the database to create a list of unique chemicals that had data available through the U.S. Environmental Protection Agency’s CompTox Dashboard and were in two or more individual samples at concentrations above the method detection limit.