THE EFFECTS of SEVERAL PAPER CHARACTERISTICS and APPLICATION METHODS on the SUBLIMATION RATE of CYCLODODECANE by Kelli A. Piot
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2013/0157779 A1 Kim Et Al
US 2013 O157779 A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0157779 A1 Kim et al. (43) Pub. Date: Jun. 20, 2013 (54) GOLF BALL COMPOSITION cover layer. The outer cover layer and/or one or more inter mediate layers includes a blend of a)2 to 60 wt % (based on (75) Inventors: Hyun J. Kim, Carlsbad, CA (US); Hong the total weight of the blend composition) of a cyclic olefin G. Jeon, Carlsbad, CA (US) polymer which includes a norbornene-based monomer hav (73) Assignee: Taylor Made Golf Company, Inc. ing the general formula: (21) Appl. No.: 13/330,453 (22) Filed: Dec. 19, 2011 Publication Classification (51) Int. Cl. A63B 37/00 (2006.01) A63B 37/2 (2006.01) R18 R19, R2 A63B 37/08 (2006.01) R20 A63B 37/02 (2006.01) A63B 37/06 (2006.01) (52) U.S. Cl. where R' to R' independently representahydrogenatom, a USPC ........... 473/373; 473/378; 473/385; 473/372; halogenatom or a hydrocarbon group and mis from 0 to about 473/376; 473/377 3; and b) about 40 to about 98 wt % (based on the total weight of the blend composition) of one or more additional polymer (57) ABSTRACT components. The blend composition has a melt flow index This invention pertains to a golf ball which includes a core, (MFI) from 1 to 80 g/10 min., a material hardness of 30 to 90 optionally one or more intermediate layers; and an outer Shore D, and a flex modulus of 10 to 120 kpsi. -

Synthesis of Functionalized Polyamide 6 by Anionic Ring-Opening Polymerization Deniz Tunc
Synthesis of functionalized polyamide 6 by anionic ring-opening polymerization Deniz Tunc To cite this version: Deniz Tunc. Synthesis of functionalized polyamide 6 by anionic ring-opening polymerization. Poly- mers. Université de Bordeaux; Université de Liège, 2014. English. NNT : 2014BORD0178. tel- 01281327 HAL Id: tel-01281327 https://tel.archives-ouvertes.fr/tel-01281327 Submitted on 2 Mar 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Logo Université de cotutelle THÈSE PRÉSENTÉE POUR OBTENIR LE GRADE DE DOCTEUR DE L’UNIVERSITÉ DE BORDEAUX ET DE L’UNIVERSITÉ DE LIEGE ÉCOLE DOCTORALEDE SCIENCES CHIMIQUES (Université de Bordeaux) ÉCOLE DOCTORALE DE CHIMIE (Université de Liège) SPÉCIALITÉ POLYMERES Par Deniz TUNC Synthesis of functionalized polyamide 6 by anionic ring-opening polymerization Sous la direction de Stéphane CARLOTTI et Philippe LECOMTE Soutenue le 30 octobre 2014 Membres du jury: M. PERUCH, Frédéric Directeur de recherche, Université de Bordeaux Président M. HOOGENBOOM, Richard Professeur, Ghent University Rapporteur M. MONTEIL, Vincent Chargé de recherche, Université Claude Bernard Rapporteur M. YAGCI, Yusuf Professeur, Istanbul Technical University Examinateur M. AMEDURI, Bruno Directeur de recherche, Institut Charles Gerhardt Examinateur M. SERVANT, Laurent Professeur, Université de Bordeaux Invité Preamble This PhD had been performed within the framework of the IDS FunMat joint doctoral programme. -

172 Subpart C—Substances for Use As Components
§ 175.210 21 CFR Ch. I (4–1–13 Edition) not to exceed 0.05 percent by weight of Rubber hydrochloride. the finished pressure-sensitive adhe- Rubber (natural latex solids or crepe, sive. smoked or unsmoked). (8) 2-Hydroxy-1-[4-(2-hydroxyethoxy) Terpene resins (a- and b-pinene), homopolymers, copolymers, and conden- phenyl]-2-methyl-1-propanone (CAS sates with phenol, formaldehyde, cou- Reg. No. 106797–53–9) as a photoinitiator marone, and/or indene. at a level not to exceed 5 percent by Tetrasodium ethylenediaminetetraacetate. weight of the pressure-sensitive adhe- Tri(mixed mono- and dinonylphenyl) sive. phosphite (which may contain not more than 1 percent by weight of (9) Butanedioic acid, sulfo-1,4-di-(C9- triisopropanolamine). C11 alkyl) ester, ammonium salt (also known as butanedioic acid sulfo-1, 4- (c) Acrylonitrile copolymers identi- diisodecyl ester, ammonium salt [CAS fied in this section shall comply with Reg. No. 144093–88–9]) as a surface ac- the provisions of § 180.22 of this chap- tive agent at a level not to exceed 3.0 ter. percent by weight of the finished pres- sure-sensitive adhesive. [42 FR 14534, Mar. 15, 1977, as amended at 42 FR 15674, Mar. 22, 1977; 48 FR 15617, Apr. 12, (b) Pressure-sensitive adhesives pre- 1983; 63 FR 3464, Jan. 23, 1998; 63 FR 51528, pared from one or a mixture of two or Sept. 28, 1998; 64 FR 48291, Sept. 3, 1999] more of the substances listed in this paragraph may be used as the food-con- Subpart C—Substances for Use as tact surface of labels and/or tapes ap- plied to raw fruit and raw vegetables. -

Tepzz¥ Z89 7A T
(19) TZZ¥ Z _T (11) EP 3 208 927 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 153(4) EPC (43) Date of publication: (51) Int Cl.: 23.08.2017 Bulletin 2017/34 H02M 7/12 (2006.01) C07C 251/44 (2006.01) H01L 33/00 (2010.01) C07B 61/00 (2006.01) (2006.01) (2006.01) (21) Application number: 15849932.7 C07D 201/04 C07D 223/10 C07D 225/02 (2006.01) (22) Date of filing: 18.09.2015 (86) International application number: PCT/JP2015/076613 (87) International publication number: WO 2016/059942 (21.04.2016 Gazette 2016/16) (84) Designated Contracting States: • TAKAHASHI, Toru AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Otsu-shi GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Shiga 520-8558 (JP) PL PT RO RS SE SI SK SM TR • ITO, Hiroyasu Designated Extension States: Nagoya-shi BA ME Aichi 4558502 (JP) Designated Validation States: • SUGAWARA, Kazuki MA Tokai-shi Aichi 476-8567 (JP) (30) Priority: 15.10.2014 JP 2014210567 (74) Representative: Hoefer & Partner Patentanwälte (71) Applicant: Toray Industries, Inc. mbB Tokyo 103-8666 (JP) Pilgersheimer Straße 20 81543 München (DE) (72) Inventors: • OHNO, Fumikatsu Otsu-shi Shiga 5208558 (JP) (54) THREE-PHASE AC/DC CONVERSION DEVICE, PHOTOCHEMICAL REACTION DEVICE AND METHOD USING SAME, AND METHOD FOR PRODUCING LACTAM (57) Provided are a three-phase AC/DC converter means outputs the pulse width modulation signals based disposed between a three-phase AC power supply and on a power supply voltage phase and an output voltage a light emitting diode group, the converter comprising: a between the DC buses, a photochemical reaction device three-phase full bridge circuit in which pairs of switching and a photochemical reaction method using the device, elements are connected in parallel between DC buses and a method for producing lactam using the photochem- for the three phases of the three-phase AC power supply; ical reaction method. -

Certified Reference Materials for Food Packaging Specific Migration Tests: Development, Validation and Modelling
Certified reference materials for food packaging specific migration tests: development, validation and modelling Niels H. Stoffers Promotor Prof. dr. ir. M.A.J.S. van Boekel Hoogleraar Productontwerpen en Kwaliteitskunde, Wageningen Universiteit. Co-promotoren Dr. ir. M. Dekker Universitair Hoofddocent bij de leerstoelgroep Productontwerpen en Kwaliteitskunde, Wageningen Universiteit Dr. ir. J.P.H. Linssen Universitair docent bij de leerstoelgroep Productontwerpen en Kwaliteitskunde, Wageningen Universiteit Samenstelling promotiecommissie Prof. dr. ir. A.G.J. Voragen (Wageningen Universiteit) Prof. dr. ir. R.M. Boom (Wageningen Universiteit) Prof. dr. ir. G. van Straten (Wageningen Universiteit) Dr. R. Franz (Fraunhofer Institut IVV, Freising, Duitsland) Dit onderzoek is uitgevoerd binnen de onderzoekschool VLAG Certified reference materials for food packaging specific migration tests: development, validation and modelling Niels H. Stoffers Proefschrift ter verkrijging van de graad van doctor op gezag van de rector magnificus van Wageningen Universiteit, Prof. dr. ir. L. Speelman in het openbaar te verdedigen op dinsdag 18 januari 2005 des namiddags te half twee in de Aula ISBN: 90-8504-136-8 Abstract Stoffers, N.H. (2004). Certified reference materials for food packaging specific migration tests: development, validation and modelling. PhD thesis, Wageningen University, The Netherlands. This thesis compiles several research topics during a feasibility study for the certification of 6 reference materials for specific migration testing of food packaging materials. The overall results of the certification exercise, covering results for 3 certification parameters (initial concentration of migrants, specific migration value and diffusion coefficient) from 4 participating laboratories were evaluated. The development and validation of analytical methods for the nylon 12 monomer laurolactam was described. -

The Influence of Electron Beam Irradiation on the Property Behaviour of Medical Grade Poly (Ether-Block-Amide) (PEBA)
Australian Journal of Basic and Applied Sciences, 7(5): 174-181, 2013 ISSN 1991-8178 The Influence Of Electron Beam Irradiation On The Property Behaviour Of Medical Grade Poly (Ether-Block-Amide) (PEBA) 1Kieran A. Murray, 1James E.Kennedy, 2Brian McEvoy, 2Olivier Vrain, 2Damien Ryan, 2Richard Cowman and 1Clement L. Higginbotham 1Materials Research Institute, Athlone Institute of Technology, Dublin Road, Athlone, Co. Westmeath, Ireland 2 Synergy Health Applied Sterilisation Technologies, IDA Business & Technology Park, Sragh, Tullamore, Co. Offaly, Ireland Abstract: The aim of this paper is to investigate the potential effects of electron beam, ranging between 5 and 200kGy for unstabilised Poly (ether-block-amide) (Pebax) material. The Pebax samples were subjected to a wide range of extensive characterisation techniques in order to identify any modifications to the material properties. The results demonstrated that a series of changes occurred and some of these included a reduction in the mechanical properties and melting strength, an increase in the molecular weight and viscosity and alterations to surface characteristics of the material. Overall, this study provided evidence that electron beam irradiation lead to the development of simultaneous crosslinking and chain scission in the Pebax material, where crosslinking/branching became the dominating factor above 10kGy. Key words: Electron beam irradiation,Poly(ether-block-amide), Thermal, mechanical, structural and surface properties, Temperature ramp, Degradation INTRODUCTION Poly (ether-block-amide) (Pebax) is a copolymer which consists of linear chains of rigid polyamide blocks covalently linked to flexible polyether blocks via ester groups (Joseph and Flesher, 1986). The polyamide crystalline region provides the mechanical strength while the polyether amorphous region offers high permeability as a result of the high chain mobility of the ether linkage (Joseph and Flesher, 1986; Liu, 2008). -
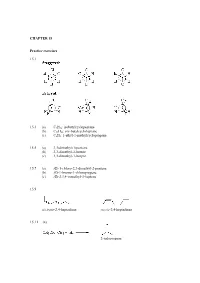
CHAPTER 15 Practice Exercises 15.1 15.3 (A) C9H18
CHAPTER 15 Practice exercises 15.1 15.3 (a) C9H18: isobutylcyclopentane (b) C11H22: sec-butylcycloheptane (c) C6H2: 1-ethyl-1-methylcyclopropane 15.5 (a) 3,3-dimethyl-1-pentene (b) 2,3-dimethyl-2-butene (c) 3,3-dimethyl-1-butyne 15.7 (a) (E)-1-chloro-2,3-dimethyl-2-pentene (b) (Z)-1-bromo-1-chloropropene (c) (E)-2,3,4-trimethyl-3-heptene 15.9 cis,trans-2,4-heptadiene cis,cis-2,4-heptadiene 15.11 (a) 2-iodopropane (b) 1-iodo-1-methylcyclohexane 15.13 CH3 CH3 + HI I Step 1: Protonation of the alkene to give the most stable 3˚ carbocation intermediate: slow step + CH3 HI CH3 I rate-determining step Step 2: Nucleophilic attack of the iodide anion on the 3˚ carbocation intermediate to give the product: CH + 3 CH3 I I 15.15 H2O CH3 CH3 + H3O OH Step 1: Protonation of the alkene to give the most stable 3˚ carbocation intermediate: slow step H + + CH3 HOH CH3 O H rate-determining H step Step 2: Nucleophilic attack of the water on the 3˚ carbocation intermediate to give the protonated alcohol: H H O+ H + CH3 O H CH3 Step 3: The protonated alcohol loses a proton to form the product: H + O H H CH3 + O + H3O CH3 H OH 15.17 (a) 2-phenyl-2-propanol (b) (E)-3,4-diphenyl-3-hexene (c) 3-methylbenzoic acid or m-methylbenzoic acid Review questions 15.1 A hydrocarbon is a compound composed only of hydrogen and carbon atoms. 15.3 In saturated hydrocarbons, each carbon is bonded to four other atoms, either hydrogen or carbon atoms. -

The Dynisco Extrusion Processors Handbook 2Nd Edition
The Dynisco Extrusion Processors Handbook 2nd edition Written by: John Goff and Tony Whelan Edited by: Don DeLaney Acknowledgements We would like to thank the following people for their contributions to this latest edition of the DYNISCO Extrusion Processors Handbook. First of all, we would like to thank John Goff and Tony Whelan who have contributed new material that has been included in this new addition of their original book. In addition, we would like to thank John Herrmann, Jim Reilly, and Joan DeCoste of the DYNISCO Companies and Christine Ronaghan and Gabor Nagy of Davis-Standard for their assistance in editing and publication. For the fig- ures included in this edition, we would like to acknowledge the contributions of Davis- Standard, Inc., Krupp Werner and Pfleiderer, Inc., The DYNISCO Companies, Dr. Harold Giles and Eileen Reilly. CONTENTS SECTION 1: INTRODUCTION TO EXTRUSION Single-Screw Extrusion . .1 Twin-Screw Extrusion . .3 Extrusion Processes . .6 Safety . .11 SECTION 2: MATERIALS AND THEIR FLOW PROPERTIES Polymers and Plastics . .15 Thermoplastic Materials . .19 Viscosity and Viscosity Terms . .25 Flow Properties Measurement . .28 Elastic Effects in Polymer Melts . .30 Die Swell . .30 Melt Fracture . .32 Sharkskin . .34 Frozen-In Orientation . .35 Draw Down . .36 SECTION 3: TESTING Testing and Standards . .37 Material Inspection . .40 Density and Dimensions . .42 Tensile Strength . .44 Flexural Properties . .46 Impact Strength . .47 Hardness and Softness . .48 Thermal Properties . .49 Flammability Testing . .57 Melt Flow Rate . .59 Melt Viscosity . .62 Measurement of Elastic Effects . .64 Chemical Resistance . .66 Electrical Properties . .66 Optical Properties . .68 Material Identification . .70 SECTION 4: THE SCREW AND BARREL SYSTEM Materials Handling . -

In This Handout, All of Our Functional Groups Are Presented As Condensed Line Formulas, 2D and 3D Formulas and with Nomenclature Prefixes and Suffixes (If Present)
In this handout, all of our functional groups are presented as condensed line formulas, 2D and 3D formulas and with nomenclature prefixes and suffixes (if present). Organic names are built on a foundation of alkanes, alkenes and alkynes. Those examples are presented first and you need to know those rules. The strategies can be found in Chapter 4 of our textbook (alkanes: pages 93-98, cycloalkanes 102-104, alkenes: pages 104-110, alkynes: pages 112-113 and combinations of all of them 113-115). After introducing examples of alkanes, alkenes, alkynes and combinations of them, the functional groups are presented in order of priority. A few nomenclature examples are provided for each of the functional groups. Examples of the various functional groups are presented on pages 115-135 in the textbook. Two overview pages are on pages 136-137. Some functional groups have a suffix name when they are the highest priority functional group and a prefix name when they are not the highest priority group, and these are added to the skeletal names with identifying numbers and stereochemistry terms (E and Z for alkenes, R and S for chiral centers and cis and trans for rings). Several low priority functional groups only have a prefix name. A few additional special patterns are shown on pages 98-102. The only way to learn this topic is practice (over and over). The best practice approach is to actually write out the names (on an extra piece of paper or on a white board, and then do it again). The same functional groups are used throughout the entire course. -

Reactivity Features of Cyclododecanone
Reviews and Accounts ARKIVOC 2011 (i) 429-495 Reactivity features of cyclododecanone Hanafi H. Zoorob, Mohamed S. Elsherbini, and Wafaa S. Hamama* Chemistry Department, Faculty of Science, Mansoura University, El-Gomhoria Street, 35516 Mansoura, Egypt E-mail: [email protected] Abstract Cyclododecanone is a highly important synthetic intermediate for macrocyclic systems. This review is the sole and comprehensive one that covers the different aspects of cyclododecanone chemistry over the period from 1950 to 2010. Keywords: Cyclododecanone, muscone, fragrances, macrocylic ketones, ring enlargement and heterocycles Table of Contents 1. Introduction 2. Synthesis of Cyclododecanone 3. Chemistry of Cyclododecanone 3.1. Halogenation 3.2. Oxidation 3.3. Alkylation 3.4. Mannich reaction 3.5. Ring enlargement 3.5.1. Macrocyclic ketones and esters 3.5.2. Macrocyclic lactames and lactones 3.5.3. Macrocyclic Acetylenes 3.6. Ring contraction 3.7. Construction of spiro and fused heterocyclic systems 3.7.1. Spiro heterocyclic systems 3.7.2. Fused heterocyclic systems 3.8. Photochemistry 3.9. Miscellaneous reactions 4. Conformational Characteristics of Cyclododecanone 5. Conclusion Page 429 ©ARKAT-USA, Inc. Reviews and Accounts ARKIVOC 2011 (i) 429-495 6. Appendix: Abbreviations and Acronyms 7. References 1. Introduction Cyclododecanone 1 is a commercially available ketone, and important synthetic intermediate in the synthesis of natural products containing macrocyclic ring systems such as the anticancer rosphellin 2,1 the potent microglial activation modulators tocopherol fatty alcohols (TFAs) 3,2 cytotoxic sponge alkaloids motuporamine A 4, motuporamine B 5,3 ingenes 6 and 7,4,5 (Scheme 1). N RO O H3CO (CH2)n O O OH NH R=H,Me,Ac Cl n=11to15 Cyclododecanone roseophilin TFAs 1 23 H N N N H2N N N 2 H H Motuporamine A Motuporamine B 4 5 O O O O O O 6a 6b 7 Scheme 1 Cyclododecanone is also an important intermediate for synthesis of natural muscone 8 and macrocyclic fragrances of musk like odor e.g. -

(12) United States Patent (10) Patent No.: US 6,649,757 B2 Kuroda Et Al
USOO6649757B2 (12) United States Patent (10) Patent No.: US 6,649,757 B2 Kuroda et al. (45) Date of Patent: Nov. 18, 2003 (54) PROCESS FOR PRODUCING FOREIGN PATENT DOCUMENTS LAUROLACTAM FROM CYCLODODECANONE CH 532 OS3 A 12/1972 EP O 487 090 A 5/1992 (75) Inventors: Nobuyuki Kuroda, Ube (JP); Joji EP O 785 188 A 7/1997 Kawai, Ube (JP); Hideo Shimomura, JP B43 12153 5/1968 Ube (JP) JP B48 10475 4/1973 (73) Assignee: Ube Industries, Ltd., Ube (JP) OTHER PUBLICATIONS * ) Notice: Subject to anyy disclaimer, the term of this patent is extended or adjusted under 35 Ritz, J. et al., “Caprolactam” Ullmann's Encyclopedia of U.S.C. 154(b) by 0 days. Industrial Chemistry. Cancer Chemotherapy to Ceramic Colorants, Weinheim, VCH Verlag, DE, vol. 15, 1986, pp. (21) Appl. No.: 10/345,548 31-50, XP002909167. (22) Filed: Jan. 16, 2003 (65) Prior Publication Data Primary Examiner Bruck Kifle US 2003/0139596 A1 Jul. 24, 2003 (74) Attorney, Agent, or Firm- Piper Rudnick LLP (30) Foreign Application Priority Data (57) ABSTRACT Jan. 16, 2002 (JP) ....................................... 2002-007549 Laurolactam having high quality is produced by reacting Feb. 28, 2002 (JP) ...... ... 2002-053903 cyclododecanone with a hydroxylamine Salt of a mineral Feb. 28, 2002 (JP) ...... ... 2002-053931 Mar. 29, 2002 (JP) ...... 2002-095O24 acid, and converting the resultant cyclododecanoneoxime to Jul. 18, 2002 (JP) r 2002-209935 laurolactam through the Beckmann rearrangement reaction, wherein a content of each of oxygen atom-containing C2 (51) Int. Cl. .............................................. C07D 225/02 organic compounds, for example, cyclododecenone or (52) U.S. -

United States Patent (19) 11 Patent Number: 5,951,941 Wang Et Al
USOO5951941A United States Patent (19) 11 Patent Number: 5,951,941 Wang et al. (45) Date of Patent: Sep. 14, 1999 54) BLOCK COPOLYMER ELASTOMER FOREIGN PATENT DOCUMENTS CATHETER BALLOONS 0 485 903 5/1982 European Pat. Off.. 75 Inventors: Lixiao Wang, Maple Grove; Jianhua O 5. 3. 3.1988/1989 European Pat. Off.. Chen, Brooklyn Park, both of Minn. 420 488 3/1991 European Pat. Off.. O 436 501 7/1991 European Pat. Off.. (73) Assignee: Scimed Life Systems, Inc., Maple O 513 459 A1 11/1992 European Pat. Off.. Grove, Minn. 540858 5/1993 European Pat. Off.. 420 488 7/1993 European Pat. Off.. O 566 755 A1 10/1993 European Pat. Off.. 21 Appl. No.: 09/137,658 531 117 B1 10/1993 European Pat. Off.. 22 Filed: Aug. 21, 1998 566 755 10/1993 European Pat. Off.. 540858 12/1993 European Pat. Off.. Related U.S. Application Data (List continued on next page.) 62 Division of application No. 08/805,899, Feb. 25, 1997, Pat. OTHER PUBLICATIONS No. 5,830,182, which is a continuation of application No. ation08/671,861, of application Jun. 28, No. 1996, 08/397,837, abandoned, Mar. which 2, 1995, is a continu-Pat. No. R 66 Elyst block amide. highinth- prismance TPE, ss 5,556,383, which is a continuation-in-part of application No. Modern Plastics, Sep. 1987, pp. 100,105,110. 08/204,554, Mar. 2, 1994, abandoned. (List continued on next page.) 51) Int. Cl. ........................... B29C 39/02; A61M 29/00 52 U.S. Cl. .............................. 264/523; 604/96; 604/97; Primary Examiner Samuel A.