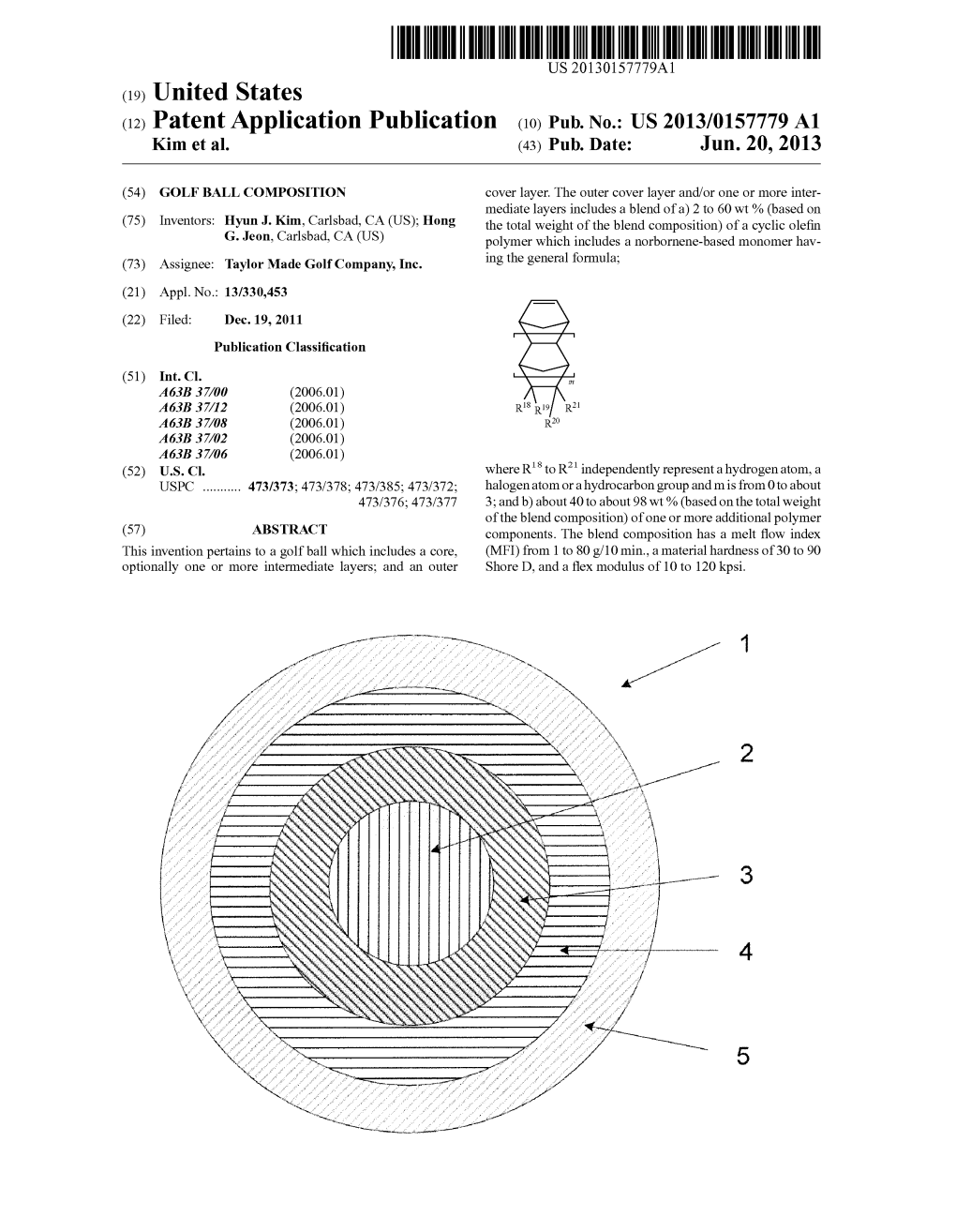
(12) Patent Application Publication (10) Pub. No.: US 2013/0157779 A1 Kim Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Synthesis of Functionalized Polyamide 6 by Anionic Ring-Opening Polymerization Deniz Tunc
Synthesis of functionalized polyamide 6 by anionic ring-opening polymerization Deniz Tunc To cite this version: Deniz Tunc. Synthesis of functionalized polyamide 6 by anionic ring-opening polymerization. Poly- mers. Université de Bordeaux; Université de Liège, 2014. English. NNT : 2014BORD0178. tel- 01281327 HAL Id: tel-01281327 https://tel.archives-ouvertes.fr/tel-01281327 Submitted on 2 Mar 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Logo Université de cotutelle THÈSE PRÉSENTÉE POUR OBTENIR LE GRADE DE DOCTEUR DE L’UNIVERSITÉ DE BORDEAUX ET DE L’UNIVERSITÉ DE LIEGE ÉCOLE DOCTORALEDE SCIENCES CHIMIQUES (Université de Bordeaux) ÉCOLE DOCTORALE DE CHIMIE (Université de Liège) SPÉCIALITÉ POLYMERES Par Deniz TUNC Synthesis of functionalized polyamide 6 by anionic ring-opening polymerization Sous la direction de Stéphane CARLOTTI et Philippe LECOMTE Soutenue le 30 octobre 2014 Membres du jury: M. PERUCH, Frédéric Directeur de recherche, Université de Bordeaux Président M. HOOGENBOOM, Richard Professeur, Ghent University Rapporteur M. MONTEIL, Vincent Chargé de recherche, Université Claude Bernard Rapporteur M. YAGCI, Yusuf Professeur, Istanbul Technical University Examinateur M. AMEDURI, Bruno Directeur de recherche, Institut Charles Gerhardt Examinateur M. SERVANT, Laurent Professeur, Université de Bordeaux Invité Preamble This PhD had been performed within the framework of the IDS FunMat joint doctoral programme. -

172 Subpart C—Substances for Use As Components
§ 175.210 21 CFR Ch. I (4–1–13 Edition) not to exceed 0.05 percent by weight of Rubber hydrochloride. the finished pressure-sensitive adhe- Rubber (natural latex solids or crepe, sive. smoked or unsmoked). (8) 2-Hydroxy-1-[4-(2-hydroxyethoxy) Terpene resins (a- and b-pinene), homopolymers, copolymers, and conden- phenyl]-2-methyl-1-propanone (CAS sates with phenol, formaldehyde, cou- Reg. No. 106797–53–9) as a photoinitiator marone, and/or indene. at a level not to exceed 5 percent by Tetrasodium ethylenediaminetetraacetate. weight of the pressure-sensitive adhe- Tri(mixed mono- and dinonylphenyl) sive. phosphite (which may contain not more than 1 percent by weight of (9) Butanedioic acid, sulfo-1,4-di-(C9- triisopropanolamine). C11 alkyl) ester, ammonium salt (also known as butanedioic acid sulfo-1, 4- (c) Acrylonitrile copolymers identi- diisodecyl ester, ammonium salt [CAS fied in this section shall comply with Reg. No. 144093–88–9]) as a surface ac- the provisions of § 180.22 of this chap- tive agent at a level not to exceed 3.0 ter. percent by weight of the finished pres- sure-sensitive adhesive. [42 FR 14534, Mar. 15, 1977, as amended at 42 FR 15674, Mar. 22, 1977; 48 FR 15617, Apr. 12, (b) Pressure-sensitive adhesives pre- 1983; 63 FR 3464, Jan. 23, 1998; 63 FR 51528, pared from one or a mixture of two or Sept. 28, 1998; 64 FR 48291, Sept. 3, 1999] more of the substances listed in this paragraph may be used as the food-con- Subpart C—Substances for Use as tact surface of labels and/or tapes ap- plied to raw fruit and raw vegetables. -

Tepzz¥ Z89 7A T
(19) TZZ¥ Z _T (11) EP 3 208 927 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 153(4) EPC (43) Date of publication: (51) Int Cl.: 23.08.2017 Bulletin 2017/34 H02M 7/12 (2006.01) C07C 251/44 (2006.01) H01L 33/00 (2010.01) C07B 61/00 (2006.01) (2006.01) (2006.01) (21) Application number: 15849932.7 C07D 201/04 C07D 223/10 C07D 225/02 (2006.01) (22) Date of filing: 18.09.2015 (86) International application number: PCT/JP2015/076613 (87) International publication number: WO 2016/059942 (21.04.2016 Gazette 2016/16) (84) Designated Contracting States: • TAKAHASHI, Toru AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Otsu-shi GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Shiga 520-8558 (JP) PL PT RO RS SE SI SK SM TR • ITO, Hiroyasu Designated Extension States: Nagoya-shi BA ME Aichi 4558502 (JP) Designated Validation States: • SUGAWARA, Kazuki MA Tokai-shi Aichi 476-8567 (JP) (30) Priority: 15.10.2014 JP 2014210567 (74) Representative: Hoefer & Partner Patentanwälte (71) Applicant: Toray Industries, Inc. mbB Tokyo 103-8666 (JP) Pilgersheimer Straße 20 81543 München (DE) (72) Inventors: • OHNO, Fumikatsu Otsu-shi Shiga 5208558 (JP) (54) THREE-PHASE AC/DC CONVERSION DEVICE, PHOTOCHEMICAL REACTION DEVICE AND METHOD USING SAME, AND METHOD FOR PRODUCING LACTAM (57) Provided are a three-phase AC/DC converter means outputs the pulse width modulation signals based disposed between a three-phase AC power supply and on a power supply voltage phase and an output voltage a light emitting diode group, the converter comprising: a between the DC buses, a photochemical reaction device three-phase full bridge circuit in which pairs of switching and a photochemical reaction method using the device, elements are connected in parallel between DC buses and a method for producing lactam using the photochem- for the three phases of the three-phase AC power supply; ical reaction method. -

Certified Reference Materials for Food Packaging Specific Migration Tests: Development, Validation and Modelling
Certified reference materials for food packaging specific migration tests: development, validation and modelling Niels H. Stoffers Promotor Prof. dr. ir. M.A.J.S. van Boekel Hoogleraar Productontwerpen en Kwaliteitskunde, Wageningen Universiteit. Co-promotoren Dr. ir. M. Dekker Universitair Hoofddocent bij de leerstoelgroep Productontwerpen en Kwaliteitskunde, Wageningen Universiteit Dr. ir. J.P.H. Linssen Universitair docent bij de leerstoelgroep Productontwerpen en Kwaliteitskunde, Wageningen Universiteit Samenstelling promotiecommissie Prof. dr. ir. A.G.J. Voragen (Wageningen Universiteit) Prof. dr. ir. R.M. Boom (Wageningen Universiteit) Prof. dr. ir. G. van Straten (Wageningen Universiteit) Dr. R. Franz (Fraunhofer Institut IVV, Freising, Duitsland) Dit onderzoek is uitgevoerd binnen de onderzoekschool VLAG Certified reference materials for food packaging specific migration tests: development, validation and modelling Niels H. Stoffers Proefschrift ter verkrijging van de graad van doctor op gezag van de rector magnificus van Wageningen Universiteit, Prof. dr. ir. L. Speelman in het openbaar te verdedigen op dinsdag 18 januari 2005 des namiddags te half twee in de Aula ISBN: 90-8504-136-8 Abstract Stoffers, N.H. (2004). Certified reference materials for food packaging specific migration tests: development, validation and modelling. PhD thesis, Wageningen University, The Netherlands. This thesis compiles several research topics during a feasibility study for the certification of 6 reference materials for specific migration testing of food packaging materials. The overall results of the certification exercise, covering results for 3 certification parameters (initial concentration of migrants, specific migration value and diffusion coefficient) from 4 participating laboratories were evaluated. The development and validation of analytical methods for the nylon 12 monomer laurolactam was described. -

Reactivity Features of Cyclododecanone
Reviews and Accounts ARKIVOC 2011 (i) 429-495 Reactivity features of cyclododecanone Hanafi H. Zoorob, Mohamed S. Elsherbini, and Wafaa S. Hamama* Chemistry Department, Faculty of Science, Mansoura University, El-Gomhoria Street, 35516 Mansoura, Egypt E-mail: [email protected] Abstract Cyclododecanone is a highly important synthetic intermediate for macrocyclic systems. This review is the sole and comprehensive one that covers the different aspects of cyclododecanone chemistry over the period from 1950 to 2010. Keywords: Cyclododecanone, muscone, fragrances, macrocylic ketones, ring enlargement and heterocycles Table of Contents 1. Introduction 2. Synthesis of Cyclododecanone 3. Chemistry of Cyclododecanone 3.1. Halogenation 3.2. Oxidation 3.3. Alkylation 3.4. Mannich reaction 3.5. Ring enlargement 3.5.1. Macrocyclic ketones and esters 3.5.2. Macrocyclic lactames and lactones 3.5.3. Macrocyclic Acetylenes 3.6. Ring contraction 3.7. Construction of spiro and fused heterocyclic systems 3.7.1. Spiro heterocyclic systems 3.7.2. Fused heterocyclic systems 3.8. Photochemistry 3.9. Miscellaneous reactions 4. Conformational Characteristics of Cyclododecanone 5. Conclusion Page 429 ©ARKAT-USA, Inc. Reviews and Accounts ARKIVOC 2011 (i) 429-495 6. Appendix: Abbreviations and Acronyms 7. References 1. Introduction Cyclododecanone 1 is a commercially available ketone, and important synthetic intermediate in the synthesis of natural products containing macrocyclic ring systems such as the anticancer rosphellin 2,1 the potent microglial activation modulators tocopherol fatty alcohols (TFAs) 3,2 cytotoxic sponge alkaloids motuporamine A 4, motuporamine B 5,3 ingenes 6 and 7,4,5 (Scheme 1). N RO O H3CO (CH2)n O O OH NH R=H,Me,Ac Cl n=11to15 Cyclododecanone roseophilin TFAs 1 23 H N N N H2N N N 2 H H Motuporamine A Motuporamine B 4 5 O O O O O O 6a 6b 7 Scheme 1 Cyclododecanone is also an important intermediate for synthesis of natural muscone 8 and macrocyclic fragrances of musk like odor e.g. -

THE EFFECTS of SEVERAL PAPER CHARACTERISTICS and APPLICATION METHODS on the SUBLIMATION RATE of CYCLODODECANE by Kelli A. Piot
THE EFFECTS OF SEVERAL PAPER CHARACTERISTICS AND APPLICATION METHODS ON THE SUBLIMATION RATE OF CYCLODODECANE by Kelli A. Piotrowski A thesis submitted to the Department of Art In conformity with the requirements for the degree of Master of Art Conservation Queen’s University Kingston, Ontario, Canada (September, 2013) Copyright © Kelli Piotrowski, 2013 ABSTRACT Cyclododecane (CDD) is a waxy, solid cyclic hydrocarbon (C12H24) that sublimes at room temperature and possesses strong hydrophobicity. In paper conservation CDD is used principally as a temporary fixative of water-soluble media during aqueous treatments. Hydrophobicity, ease of reversibility, low toxicity, and absence of residues are reasons often cited for its use over alternative materials although the latter two claims continue to be debated in the literature. The sublimation rate has important implications for treatment planning as well as health and safety considerations given the dearth of reliable information on its toxicity and exposure limits. This study examined how the rate of sublimation is affected by fiber type, sizing, and surface finish as well as delivery in the molten phase and as a saturated solution in low boiling petroleum ether. The effect of warming the paper prior to application was also evaluated. Sublimation was monitored using gravimetric analysis after which samples were tested for residues with gas chromatography-flame ionization detection (GC-FID) to confirm complete sublimation. Water absorbency tests were conducted to determine whether this property is fully reestablished. Results suggested that the sublimation rate of CDD is affected minimally by all of the paper characteristics and application methods examined in this study. The main factors influencing the rate appear to be the thickness and mass of the CDD over a given surface area as well as temperature and ventilation. -

(12) United States Patent (10) Patent No.: US 6,649,757 B2 Kuroda Et Al
USOO6649757B2 (12) United States Patent (10) Patent No.: US 6,649,757 B2 Kuroda et al. (45) Date of Patent: Nov. 18, 2003 (54) PROCESS FOR PRODUCING FOREIGN PATENT DOCUMENTS LAUROLACTAM FROM CYCLODODECANONE CH 532 OS3 A 12/1972 EP O 487 090 A 5/1992 (75) Inventors: Nobuyuki Kuroda, Ube (JP); Joji EP O 785 188 A 7/1997 Kawai, Ube (JP); Hideo Shimomura, JP B43 12153 5/1968 Ube (JP) JP B48 10475 4/1973 (73) Assignee: Ube Industries, Ltd., Ube (JP) OTHER PUBLICATIONS * ) Notice: Subject to anyy disclaimer, the term of this patent is extended or adjusted under 35 Ritz, J. et al., “Caprolactam” Ullmann's Encyclopedia of U.S.C. 154(b) by 0 days. Industrial Chemistry. Cancer Chemotherapy to Ceramic Colorants, Weinheim, VCH Verlag, DE, vol. 15, 1986, pp. (21) Appl. No.: 10/345,548 31-50, XP002909167. (22) Filed: Jan. 16, 2003 (65) Prior Publication Data Primary Examiner Bruck Kifle US 2003/0139596 A1 Jul. 24, 2003 (74) Attorney, Agent, or Firm- Piper Rudnick LLP (30) Foreign Application Priority Data (57) ABSTRACT Jan. 16, 2002 (JP) ....................................... 2002-007549 Laurolactam having high quality is produced by reacting Feb. 28, 2002 (JP) ...... ... 2002-053903 cyclododecanone with a hydroxylamine Salt of a mineral Feb. 28, 2002 (JP) ...... ... 2002-053931 Mar. 29, 2002 (JP) ...... 2002-095O24 acid, and converting the resultant cyclododecanoneoxime to Jul. 18, 2002 (JP) r 2002-209935 laurolactam through the Beckmann rearrangement reaction, wherein a content of each of oxygen atom-containing C2 (51) Int. Cl. .............................................. C07D 225/02 organic compounds, for example, cyclododecenone or (52) U.S. -

162 Part 175—Indirect Food Addi
§ 174.6 21 CFR Ch. I (4–1–19 Edition) (c) The existence in this subchapter B Subpart B—Substances for Use Only as of a regulation prescribing safe condi- Components of Adhesives tions for the use of a substance as an Sec. article or component of articles that 175.105 Adhesives. contact food shall not be construed as 175.125 Pressure-sensitive adhesives. implying that such substance may be safely used as a direct additive in food. Subpart C—Substances for Use as (d) Substances that under conditions Components of Coatings of good manufacturing practice may be 175.210 Acrylate ester copolymer coating. safely used as components of articles 175.230 Hot-melt strippable food coatings. that contact food include the fol- 175.250 Paraffin (synthetic). lowing, subject to any prescribed limi- 175.260 Partial phosphoric acid esters of pol- yester resins. tations: 175.270 Poly(vinyl fluoride) resins. (1) Substances generally recognized 175.300 Resinous and polymeric coatings. as safe in or on food. 175.320 Resinous and polymeric coatings for (2) Substances generally recognized polyolefin films. as safe for their intended use in food 175.350 Vinyl acetate/crotonic acid copoly- mer. packaging. 175.360 Vinylidene chloride copolymer coat- (3) Substances used in accordance ings for nylon film. with a prior sanction or approval. 175.365 Vinylidene chloride copolymer coat- (4) Substances permitted for use by ings for polycarbonate film. 175.380 Xylene-formaldehyde resins con- regulations in this part and parts 175, densed with 4,4′-isopropylidenediphenol- 176, 177, 178 and § 179.45 of this chapter. -

United States Patent Office Patented Feb
3,792,045 United States Patent Office Patented Feb. 12, 1974 1. 2 fore the production of pure lactam, especially of unsubsti 3,792,045 tuted lactams having 6-12 carbon atoms in the ring, and PROCESS FOR THE PURIFICATION OF LACTAMS in particular caprolactam, from extract lactam or another Ruthild Henn, Domat-Ems, Grisons, and Dirk Deiters, raw lactam by combined treatment of distillation under Hans-Joachim Schultze, and Clau Berthers. 9hrs reduced pressure and crystalline extraction of the lactam Grisons,Forschting Switzerland, und Patentverwertung, assignors to Zurich, Invent Switzerland AG fur with an aliphatic or cycloaliphatic hydrocarbon having No Drawing. Filed Dec. 22, 1971, Ser. No. 211,102 5-10 carbon atoms, particularly with cyclohexane or pe troleum ether. It is of no significance whether the raw Claims priority, applicatiwitzerland, Jan. 6, 1971, lactam is first distilled and then extracted in the crystalline Int, C. C07d 41/06 O state, or vice versa. The purification effect is unexpectedly U.S. C. 260-239.3 A 14 Claims equally good and the selection of the sequence of opera tions need only be suited to technical requirements. The process may advantageously be carried out continuously. ABSTRACT OF THE DISCLOSURE This simple combination of two purification stages has A process characterized in that raw caprolactam is sub 15 the following advantages over existing purification proc jected, in either sequence, to a crystal extraction with an eSSeS aliphatic or cycloaliphatic hydrocarbon having 5-10 car It avoids complicated multi-stage distillation and recti bon atoms and to distillation under reduced pressure. fication plant, requires no additional physical purification treatment, e.g. -

Staging Category Base Rate Article Description HTS Heading
HTS Heading/Article Subheading description Base Rate Staging Category I. CHEMICAL ELEMENTS 2801 Fluorine, chlorine, bromine and iodine: 28011000 -Chlorine Free E 28012000 -Iodine Free E 280130 -Fluorine; bromine: 28013010 --Fluorine 3.7% A 28013020 --Bromine 5.5% A 28020000 Sulfur, sublimed or precipitated; colloidal sulfur Free E Carbon (carbon blacks and other forms of carbon not 28030000 elsewhere specified or included) Free E 2804 Hydrogen, rare gases and other nonmetals: 28041000 -Hydrogen 3.7% A -Rare gases: 28042100 --Argon 3.7% A 28042900 --Other 3.7% A 28043000 -Nitrogen 3.7% A 28044000 -Oxygen 3.7% A 28045000 -Boron; tellurium Free E -Silicon: --Containing by weight not less than 99.99 percent of 28046100 silicon Free E 280469 --Other: ---Containing by weight less than 99.99 percent but not 28046910 less than 99 percent of silicon 5.3% B 28046950 ---Other 5.5% B 28047000 -Phosphorus Free E 28048000 -Arsenic Free E 28049000 -Selenium Free E Alkali or alkaline-earth metals; rare-earth metals, scandium and yttrium, whether or not intermixed or 2805 interalloyed; mercury: -Alkali metals: 28051100 --Sodium 5.3% B 28051900 --Other 5.5% B -Alkaline-earth metals: 28052100 --Calcium 3% A 280522 --Strontium and barium: 28052210 ---Strontium 3.7% A 28052220 ---Barium Free E -Rare-earth metals, scandium and yttrium, whether or 28053000 not intermixed or interalloyed 5% A 28054000 -Mercury 1.7% A II. INORGANIC ACIDS AND INORGANIC OXYGEN COMPOUNDS OF NONMETALS Hydrogen chloride (Hydrochloric acid); chlorosulfuric 2806 acid: 28061000 -Hydrogen -

United States Patent Oihce Patented June 22, 1971 1 2 of the Components of the Mixture in ?Nely Divided Form
3,586,068 United States Patent Oihce Patented June 22, 1971 1 2 of the components of the mixture in ?nely divided form. 3,586,668 The mixture thus prepared may either be tabletted or PROCESS FOR THE PRODUCTION OF LACTAMS Otto Immel, Krefeld-Urdingen, and Hans - Helmut pelletised. Alternatively, it may even be made into a paste Schwarz and Hermann Schuell, Krefeld, Germany, as with water or an organic liquid, for example a polyhydric signors to Farbenfabriken Bayer Aktiengesellschaft, alcohol, and the resulting paste dried. It is also possible, Leverkusen, Germany for example, to stir carbon black into a solution of boric No Drawing. Filed Feb. 23, 1968, Ser. No. 707,423 acid and to dry the resulting mixture by conventional Claims priority, applicatisoln7ggrmany, Mar. 3, 1967, methods. This may be followed by calcination, optionally in vacuo, at a temperature of from 400 to 800° C. The Int. Cl. c0’7d 41/06 10 US. Cl. 260-2395 9 Claims ratio of boric acid to carbon may be from 1:2 to 9:1, although particularly good results are obtained by rear rangement of the oximes in the presence of a catalyst in ABSTRACT OF THE DISCLOSURE which the ratio of boric acid to carbon is from 5:4 to 8:2. With one part by weight of catalyst, it is possible to re The invention relates to a process for the production of arrange 6 to 12 parts by weight of oxime. Thereafter, it lactams by catalytic rearrangement of ketoximes in the is advisable to regenerate the catalyst by heating it at a gaseous phase, using as a catalyst system a mixture of temperature from 500 to 800° C. -

COMMISSION DIRECTIVE 2002/72/EC of 6 August 2002 Relating to Plastic Materials and Articles Intended to Come Into Contact with Foodstuffs (Text with EEA Relevance)
L 220/18EN Official Journal of the European Communities 15.8.2002 COMMISSION DIRECTIVE 2002/72/EC of 6 August 2002 relating to plastic materials and articles intended to come into contact with foodstuffs (Text with EEA relevance) THE COMMISSION OF THE EUROPEAN COMMUNITIES, (8) Besides the monomers and other starting substances fully evaluated and authorised at Community level, there are Having regard to the Treaty establishing the European also monomers and starting substances evaluated and Community, authorised in at least one Member State which may continue to be used pending their evaluation by the Scientific Committee on Food and the decision on their Having regard to Council Directive 89/109/EEC of 21 inclusion in the Community list; this Directive will December 1988 on the approximation of the laws of the accordingly be extended in due course to the substances Member States relating to materials and articles intended to and sectors provisionally excluded. come into contact with foodstuffs (1), and in particular Article 3 thereof, (9) The current list of additives is an incomplete list inas- After consulting the Scientific Committee on Food, much as it does not contain all the substances which are currently accepted in one or more Member States; Whereas: accordingly, these substances continue to be regulated by national laws pending a decision on inclusion in the Community list. (1) Commission Directive 90/128/EEC of 23 February 1990 relating to plastic materials and articles intended to come into contact with foodstuffs (2), as last amended by Direc- tive 2002/17/EC (3), has been frequently and substantially (10) This Directive establishes specifications for only a few amended; for reasons of clarity and rationality, it should substances.