Reactions of OH Radicals with C6АC10 Cycloalkanes in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Implications for Extraterrestrial Hydrocarbon Chemistry: Analysis Of
The Astrophysical Journal, 889:3 (26pp), 2020 January 20 https://doi.org/10.3847/1538-4357/ab616c © 2020. The American Astronomical Society. All rights reserved. Implications for Extraterrestrial Hydrocarbon Chemistry: Analysis of Acetylene (C2H2) and D2-acetylene (C2D2) Ices Exposed to Ionizing Radiation via Ultraviolet–Visible Spectroscopy, Infrared Spectroscopy, and Reflectron Time-of-flight Mass Spectrometry Matthew J. Abplanalp1,2 and Ralf I. Kaiser1,2 1 W. M. Keck Research Laboratory in Astrochemistry, University of Hawaii at Manoa, Honolulu, HI 96822, USA 2 Department of Chemistry, University of Hawaii at Manoa, Honolulu, HI 96822, USA Received 2019 October 4; revised 2019 December 7; accepted 2019 December 10; published 2020 January 20 Abstract The processing of the simple hydrocarbon ice, acetylene (C2H2/C2D2), via energetic electrons, thus simulating the processes in the track of galactic cosmic-ray particles penetrating solid matter, was carried out in an ultrahigh vacuum surface apparatus. The chemical evolution of the ices was monitored online and in situ utilizing Fourier transform infrared spectroscopy (FTIR) and ultraviolet–visible spectroscopy and, during temperature programmed desorption, via a quadrupole mass spectrometer with an electron impact ionization source (EI-QMS) and a reflectron time-of-flight mass spectrometer utilizing single-photon photoionization (SPI-ReTOF-MS) along with resonance-enhanced multiphoton photoionization (REMPI-ReTOF-MS). The confirmation of previous in situ studies of ethylene ice irradiation -

First Principles Prediction of Thermodynamic Properties
2 First Principles Prediction of Thermodynamic Properties Hélio F. Dos Santos and Wagner B. De Almeida NEQC: Núcleo de Estudos em Química Computacional, Departamento de Química, ICE Universidade Federal de Juiz de Fora (UFJF), Campus Universitário Martelos, Juiz de Fora LQC-MM: Laboratório de Química Computacional e Modelagem Molecular Departamento de Química, ICEx, Universidade Federal de Minas Gerais (UFMG) Campus Universitário, Pampulha, Belo Horizonte Brazil 1. Introduction The determination of the molecular structure is undoubtedly an important issue in chemistry. The knowledge of the tridimensional structure allows the understanding and prediction of the chemical-physics properties and the potential applications of the resulting material. Nevertheless, even for a pure substance, the structure and measured properties reflect the behavior of many distinct geometries (conformers) averaged by the Boltzmann distribution. In general, for flexible molecules, several conformers can be found and the analysis of the physical and chemical properties of these isomers is known as conformational analysis (Eliel, 1965). In most of the cases, the conformational processes are associated with small rotational barriers around single bonds, and this fact often leads to mixtures, in which many conformations may exist in equilibrium (Franklin & Feltkamp, 1965). Therefore, the determination of temperature-dependent conformational population is very much welcomed in conformational analysis studies carried out by both experimentalists and theoreticians. -

Comparing Models for Measuring Ring Strain of Common Cycloalkanes
The Corinthian Volume 6 Article 4 2004 Comparing Models for Measuring Ring Strain of Common Cycloalkanes Brad A. Hobbs Georgia College Follow this and additional works at: https://kb.gcsu.edu/thecorinthian Part of the Chemistry Commons Recommended Citation Hobbs, Brad A. (2004) "Comparing Models for Measuring Ring Strain of Common Cycloalkanes," The Corinthian: Vol. 6 , Article 4. Available at: https://kb.gcsu.edu/thecorinthian/vol6/iss1/4 This Article is brought to you for free and open access by the Undergraduate Research at Knowledge Box. It has been accepted for inclusion in The Corinthian by an authorized editor of Knowledge Box. Campring Models for Measuring Ring Strain of Common Cycloalkanes Comparing Models for Measuring R..ing Strain of Common Cycloalkanes Brad A. Hobbs Dr. Kenneth C. McGill Chemistry Major Faculty Sponsor Introduction The number of carbon atoms bonded in the ring of a cycloalkane has a large effect on its energy. A molecule's energy has a vast impact on its stability. Determining the most stable form of a molecule is a usefol technique in the world of chemistry. One of the major factors that influ ence the energy (stability) of cycloalkanes is the molecule's ring strain. Ring strain is normally viewed as being directly proportional to the insta bility of a molecule. It is defined as a type of potential energy within the cyclic molecule, and is determined by the level of "strain" between the bonds of cycloalkanes. For example, propane has tl1e highest ring strain of all cycloalkanes. Each of propane's carbon atoms is sp3-hybridized. -

Text Related to Segment 5.02 ©2002 Claude E. Wintner from the Previous Segment We Have the Value of the Heat of Combustion Of
Text Related to Segment 5.02 ©2002 Claude E. Wintner From the previous segment we have the value of the heat of combustion of an "unstrained" methylene unit as -157.4 kcal/mole. On this basis one would predict that combustion of "unstrained" cyclopropane should liberate 3 X 157.4 = 472.2 kcal/mole; however, when cyclopropane is burned, a value of 499.8 kcal/mole is measured for the actual heat of combustion. The difference of 27.6 kcal/mole is interpreted as representing the higher internal energy ("strain energy") of real cyclopropane as compared to a postulated strain-free "model." ("Model" in this usage speaks of a proposal, or postulate.) These facts are graphed conveniently on an energy diagram as follows: "Strain Energy" represents the error units: kcal/mole not to scale in the "strain-free model" real cyclopropane + 4.5 O2 27.6 "strain-free model" of cyclopropane 499.8 observed heat of combustion + 4.5 O2 472.2 3CO2 + 3H2O heat of combustion predicted for "strain-free model" Note that this estimate of the strain energy in cyclopropane amounts to 9.2 kcal/mole of strain per methylene group (dividing 27.6 by 3, because of the three carbon atoms). Comparable values in kcal/mole for other small and medium rings are given in the following table. As one might expect, in general the strain decreases as the ring is enlarged. units: kcal/mole n Total Strain Strain per CH2 3 27.6 9.2 (CH2)n 4 26.3 6.6 5 6.2 1.2 6 0.1 0.0 ! 7 6.2 0.9 8 9.7 1.2 9 12.6 1.4 10 12.4 1.2 12 4.1 0.3 15 1.9 0.1 Without entering into a discussion of the relevant bonding concepts here, and instead relying on geometry alone, interpretation of the source of the strain energy in cyclopropane and cyclobutane is to some extent self-evident. -

Annex XV Report
Annex XV report PROPOSAL FOR IDENTIFICATION OF A SUBSTANCE OF VERY HIGH CONCERN ON THE BASIS OF THE CRITERIA SET OUT IN REACH ARTICLE 57 Substance Name(s): 1,6,7,8,9,14,15,16,17,17,18,18- Dodecachloropentacyclo[12.2.1.16,9.02,13.05,10]octadeca-7,15-diene (“Dechlorane Plus”TM) [covering any of its individual anti- and syn-isomers or any combination thereof] EC Number(s): 236-948-9; -; - CAS Number(s): 13560-89-9; 135821-74-8; 135821-03-3 Submitted by: United Kingdom Date: 29 August 2017 ANNEX XV – IDENTIFICATION OF DECHLORANE PLUS AS SVHC CONTENTS PROPOSAL FOR IDENTIFICATION OF A SUBSTANCE OF VERY HIGH CONCERN ON THE BASIS OF THE CRITERIA SET OUT IN REACH ARTICLE 57..........................................................................................IV PART I ...............................................................................................................................................1 JUSTIFICATION ..................................................................................................................................1 1. IDENTITY OF THE SUBSTANCE AND PHYSICAL AND CHEMICAL PROPERTIES ...................................2 1.1 Name and other identifiers of the substance................................................................................2 1.2 Composition of the substance ........................................................................................................2 1.3 Identity and composition of degradation products/metabolites relevant for the SVHC assessment ....................................................................................................................................3 -

Chapter 2: Alkanes Alkanes from Carbon and Hydrogen
Chapter 2: Alkanes Alkanes from Carbon and Hydrogen •Alkanes are carbon compounds that contain only single bonds. •The simplest alkanes are hydrocarbons – compounds that contain only carbon and hydrogen. •Hydrocarbons are used mainly as fuels, solvents and lubricants: H H H H H H H H H H H H C H C C H C C C C H H C C C C C H H H C C H H H H H H CH2 H CH3 H H H H CH3 # of carbons boiling point range Use 1-4 <20 °C fuel (gasses such as methane, propane, butane) 5-6 30-60 solvents (petroleum ether) 6-7 60-90 solvents (ligroin) 6-12 85-200 fuel (gasoline) 12-15 200-300 fuel (kerosene) 15-18 300-400 fuel (heating oil) 16-24 >400 lubricating oil, asphalt Hydrocarbons Formula Prefix Suffix Name Structure H CH4 meth- -ane methane H C H H C H eth- -ane ethane 2 6 H3C CH3 C3H8 prop- -ane propane C4H10 but- -ane butane C5H12 pent- -ane pentane C6H14 hex- -ane hexane C7H16 hept- -ane heptane C8H18 oct- -ane octane C9H20 non- -ane nonane C10H22 dec- -ane decane Hydrocarbons Formula Prefix Suffix Name Structure H CH4 meth- -ane methane H C H H H H C2H6 eth- -ane ethane H C C H H H H C H prop- -ane propane 3 8 H3C C CH3 or H H H C H 4 10 but- -ane butane H3C C C CH3 or H H H C H 4 10 but- -ane butane? H3C C CH3 or CH3 HydHrydorcocaarrbobnos ns Formula Prefix Suffix Name Structure H CH4 meth- -ane methane H C H H H H C2H6 eth- -ane ethane H C C H H H H C3H8 prop- -ane propane H3C C CH3 or H H H C H 4 10 but- -ane butane H3C C C CH3 or H H H C H 4 10 but- -ane iso-butane H3C C CH3 or CH3 HydHrydoroccarbrobnsons Formula Prefix Suffix Name Structure H H -
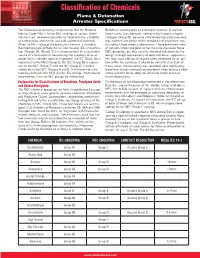
Classification of Chemicals
Classification of Chemicals Flame & Detonation Arrester Specifications PROTECTOSEAL ® The Protectoseal Company recommends that the National Butadiene would qualify as a Group D material. In each of Electric Code (NEC) Article 500, rankings of various chemi - these cases, the chemicals were primarly listed in a higher cals be used, whenever possible, to determine the suitability category (Group B), because of relatively high pressure read - of a detonation arrester for use with a particular chemical. ings noted in one phase of the standard test procedure con - When no NEC rating of the particular chemical is available, ducted by Underwriters Laboratories. These pressures were the International Electrotechnical Commission (IEC) classifica - of concern when categorizing the chemicals because these tion (Groups IIA, IIB and IIC) is recommended as a secondary NEC groupings are also used as standard indicators for the source of information for determining the suitability of an ar - design strength requirements of electrical boxes, apparatus, rester for its intended service. In general, the IEC Group IIA is etc. that must withstand the pressures generated by an igni - equivalent to the NEC Group D; the IEC Group IIB is equiva - tion within the container. It should be noted that, in each of lent to the NEC Group C; and the IEC Group IIC includes these cases, the test pressures recorded were significantly chemicals in the NEC Groups A and B. In the event of a dis - lower than those commonly encountered when testing a deto - crepancy between the NEC and the IEC ratings, Protectoseal nation arrester for its ability to withstand stable and over - recommends that the NEC groups be referenced. -

The Enthalpy of Formation of Organic Compounds with “Chemical Accuracy”
chemengineering Article Group Contribution Revisited: The Enthalpy of Formation of Organic Compounds with “Chemical Accuracy” Robert J. Meier Pro-Deo Consultant, 52525 Heinsberg, North-Rhine Westphalia, Germany; [email protected] Abstract: Group contribution (GC) methods to predict thermochemical properties are of eminent importance to process design. Compared to previous works, we present an improved group contri- bution parametrization for the heat of formation of organic molecules exhibiting chemical accuracy, i.e., a maximum 1 kcal/mol (4.2 kJ/mol) difference between the experiment and model, while, at the same time, minimizing the number of parameters. The latter is extremely important as too many parameters lead to overfitting and, therewith, to more or less serious incorrect predictions for molecules that were not within the data set used for parametrization. Moreover, it was found to be important to explicitly account for common chemical knowledge, e.g., geminal effects or ring strain. The group-related parameters were determined step-wise: first, alkanes only, and then only one additional group in the next class of molecules. This ensures unique and optimal parameter values for each chemical group. All data will be made available, enabling other researchers to extend the set to other classes of molecules. Keywords: enthalpy of formation; thermodynamics; molecular modeling; group contribution method; quantum mechanical method; chemical accuracy; process design Citation: Meier, R.J. Group Contribution Revisited: The Enthalpy of Formation of Organic Compounds with “Chemical Accuracy”. 1. Introduction ChemEngineering 2021, 5, 24. To understand chemical reactivity and/or chemical equilibria, knowledge of thermo- o https://doi.org/10.3390/ dynamic properties such as gas-phase standard enthalpy of formation DfH gas is a necessity. -

Baeyer Strain Theory Introduction Van't Hoff and Lebel Proposed
Baeyer Strain Theory instable cycloalkanes. The large ring systems involve negative strain hence do not exists. Introduction The bond angles in cyclohexane and higher Van’t Hoff and Lebel proposed tetrahedral cycloalkanes (cycloheptane, cyclooctane, geometry of carbon. The bond angel is of 109˚ cyclononane……..) are not larger than 109.5 28' (or 109.5˚) for carbon atom in tetrahedral because the carbon rings of those compounds geometry (methane molecule). Baeyer are not planar (flat) but they are puckered observed different bond angles for different (Wrinkled). cycloalkanes and also observed some These assumptions are helpful to understand different properties and stability .On this instability of cycloalkane ring systems. basis, he proposed angle strain theory. The theory explains reactivity and stability of Cyclopropane is more prone to cycloalkanes. Baeyer proposed that the undergo ring opening reaction than optimum overlap of atomic orbitals is cyclobutane or cyclopentane achieved for bond angel of 109.5 .In short, it is Cyclopropane is more reactive than ideal bond angle for alkane compounds. cyclobutane and cyclopentane Effective and optimum overlap of atomic orbitals produces maximum bond strength The ring of cyclopropane is triangle. hance stable molecule. All the three angles are of 60 in place of 109.5 (normal bond angle for If bond angles deviate from the ideal carbon atom) to adjust them into then ring produce strain. triangle ring system. Higher the strain higher the In same manner, cyclobutane is instability. square and the bond angles are of 90o in place of 109.5o (normal bond Higher strain increases reactivity and angle for carbon atom) to adjust them increases heat of combustion. -

Cycloalkanes, Cycloalkenes, and Cycloalkynes
CYCLOALKANES, CYCLOALKENES, AND CYCLOALKYNES any important hydrocarbons, known as cycloalkanes, contain rings of carbon atoms linked together by single bonds. The simple cycloalkanes of formula (CH,), make up a particularly important homologous series in which the chemical properties change in a much more dramatic way with increasing n than do those of the acyclic hydrocarbons CH,(CH,),,-,H. The cyclo- alkanes with small rings (n = 3-6) are of special interest in exhibiting chemical properties intermediate between those of alkanes and alkenes. In this chapter we will show how this behavior can be explained in terms of angle strain and steric hindrance, concepts that have been introduced previously and will be used with increasing frequency as we proceed further. We also discuss the conformations of cycloalkanes, especially cyclo- hexane, in detail because of their importance to the chemistry of many kinds of naturally occurring organic compounds. Some attention also will be paid to polycyclic compounds, substances with more than one ring, and to cyclo- alkenes and cycloalkynes. 12-1 NOMENCLATURE AND PHYSICAL PROPERTIES OF CYCLOALKANES The IUPAC system for naming cycloalkanes and cycloalkenes was presented in some detail in Sections 3-2 and 3-3, and you may wish to review that ma- terial before proceeding further. Additional procedures are required for naming 446 12 Cycloalkanes, Cycloalkenes, and Cycloalkynes Table 12-1 Physical Properties of Alkanes and Cycloalkanes Density, Compounds Bp, "C Mp, "C diO,g ml-' propane cyclopropane butane cyclobutane pentane cyclopentane hexane cyclohexane heptane cycloheptane octane cyclooctane nonane cyclononane "At -40". bUnder pressure. polycyclic compounds, which have rings with common carbons, and these will be discussed later in this chapter. -

Process for the Preparation of (11Α,13E,15S)-11,15-Dihydroxy-9-Oxoprost-13-En-1-Oic Acid
Technical Disclosure Commons Defensive Publications Series July 2020 Process for the preparation of (11α,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en-1-oic acid Srinivasan Thirumalai Rajan Follow this and additional works at: https://www.tdcommons.org/dpubs_series Recommended Citation Srinivasan Thirumalai Rajan, "Process for the preparation of (11α,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en-1-oic acid", Technical Disclosure Commons, (July 10, 2020) https://www.tdcommons.org/dpubs_series/3418 This work is licensed under a Creative Commons Attribution 4.0 License. This Article is brought to you for free and open access by Technical Disclosure Commons. It has been accepted for inclusion in Defensive Publications Series by an authorized administrator of Technical Disclosure Commons. : Process for the preparation of (11?,13E,15S)-11,15-dihydroxy-9-ox Process for the preparation of (11α,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en- 1-oic acid Field of the invention: 5 The present application relates a process for the preparation of (11α,13E,15S)- 11,15-dihydroxy-9-oxoprost-13-en-1-oic acid. Formula-1 10 Background of the invention: (11α,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en-1-oic acid is generally known as Prostaglandin E1 (PGE1) or Alprostadil. Alprostadil was approved in US and Europe under the brand name of CAVERJECT® and indicated for the treatment 15 of erectile dysfunction due to neurogenic, vasculogenic, psychogenic, or mixed etiology. Biosynthetic PGE1, is formed from dihomo-γ-linolenic acid was disclosed in Prostaglandin 20, 187, 1980. Syntheses of PGE1 was disclosed in J.