Process for the Preparation of (11Α,13E,15S)-11,15-Dihydroxy-9-Oxoprost-13-En-1-Oic Acid
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Chapter 2: Alkanes Alkanes from Carbon and Hydrogen
Chapter 2: Alkanes Alkanes from Carbon and Hydrogen •Alkanes are carbon compounds that contain only single bonds. •The simplest alkanes are hydrocarbons – compounds that contain only carbon and hydrogen. •Hydrocarbons are used mainly as fuels, solvents and lubricants: H H H H H H H H H H H H C H C C H C C C C H H C C C C C H H H C C H H H H H H CH2 H CH3 H H H H CH3 # of carbons boiling point range Use 1-4 <20 °C fuel (gasses such as methane, propane, butane) 5-6 30-60 solvents (petroleum ether) 6-7 60-90 solvents (ligroin) 6-12 85-200 fuel (gasoline) 12-15 200-300 fuel (kerosene) 15-18 300-400 fuel (heating oil) 16-24 >400 lubricating oil, asphalt Hydrocarbons Formula Prefix Suffix Name Structure H CH4 meth- -ane methane H C H H C H eth- -ane ethane 2 6 H3C CH3 C3H8 prop- -ane propane C4H10 but- -ane butane C5H12 pent- -ane pentane C6H14 hex- -ane hexane C7H16 hept- -ane heptane C8H18 oct- -ane octane C9H20 non- -ane nonane C10H22 dec- -ane decane Hydrocarbons Formula Prefix Suffix Name Structure H CH4 meth- -ane methane H C H H H H C2H6 eth- -ane ethane H C C H H H H C H prop- -ane propane 3 8 H3C C CH3 or H H H C H 4 10 but- -ane butane H3C C C CH3 or H H H C H 4 10 but- -ane butane? H3C C CH3 or CH3 HydHrydorcocaarrbobnos ns Formula Prefix Suffix Name Structure H CH4 meth- -ane methane H C H H H H C2H6 eth- -ane ethane H C C H H H H C3H8 prop- -ane propane H3C C CH3 or H H H C H 4 10 but- -ane butane H3C C C CH3 or H H H C H 4 10 but- -ane iso-butane H3C C CH3 or CH3 HydHrydoroccarbrobnsons Formula Prefix Suffix Name Structure H H -
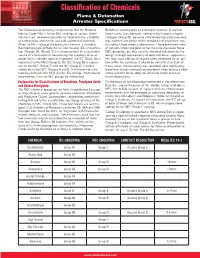
Classification of Chemicals
Classification of Chemicals Flame & Detonation Arrester Specifications PROTECTOSEAL ® The Protectoseal Company recommends that the National Butadiene would qualify as a Group D material. In each of Electric Code (NEC) Article 500, rankings of various chemi - these cases, the chemicals were primarly listed in a higher cals be used, whenever possible, to determine the suitability category (Group B), because of relatively high pressure read - of a detonation arrester for use with a particular chemical. ings noted in one phase of the standard test procedure con - When no NEC rating of the particular chemical is available, ducted by Underwriters Laboratories. These pressures were the International Electrotechnical Commission (IEC) classifica - of concern when categorizing the chemicals because these tion (Groups IIA, IIB and IIC) is recommended as a secondary NEC groupings are also used as standard indicators for the source of information for determining the suitability of an ar - design strength requirements of electrical boxes, apparatus, rester for its intended service. In general, the IEC Group IIA is etc. that must withstand the pressures generated by an igni - equivalent to the NEC Group D; the IEC Group IIB is equiva - tion within the container. It should be noted that, in each of lent to the NEC Group C; and the IEC Group IIC includes these cases, the test pressures recorded were significantly chemicals in the NEC Groups A and B. In the event of a dis - lower than those commonly encountered when testing a deto - crepancy between the NEC and the IEC ratings, Protectoseal nation arrester for its ability to withstand stable and over - recommends that the NEC groups be referenced. -

Cycloalkanes, Cycloalkenes, and Cycloalkynes
CYCLOALKANES, CYCLOALKENES, AND CYCLOALKYNES any important hydrocarbons, known as cycloalkanes, contain rings of carbon atoms linked together by single bonds. The simple cycloalkanes of formula (CH,), make up a particularly important homologous series in which the chemical properties change in a much more dramatic way with increasing n than do those of the acyclic hydrocarbons CH,(CH,),,-,H. The cyclo- alkanes with small rings (n = 3-6) are of special interest in exhibiting chemical properties intermediate between those of alkanes and alkenes. In this chapter we will show how this behavior can be explained in terms of angle strain and steric hindrance, concepts that have been introduced previously and will be used with increasing frequency as we proceed further. We also discuss the conformations of cycloalkanes, especially cyclo- hexane, in detail because of their importance to the chemistry of many kinds of naturally occurring organic compounds. Some attention also will be paid to polycyclic compounds, substances with more than one ring, and to cyclo- alkenes and cycloalkynes. 12-1 NOMENCLATURE AND PHYSICAL PROPERTIES OF CYCLOALKANES The IUPAC system for naming cycloalkanes and cycloalkenes was presented in some detail in Sections 3-2 and 3-3, and you may wish to review that ma- terial before proceeding further. Additional procedures are required for naming 446 12 Cycloalkanes, Cycloalkenes, and Cycloalkynes Table 12-1 Physical Properties of Alkanes and Cycloalkanes Density, Compounds Bp, "C Mp, "C diO,g ml-' propane cyclopropane butane cyclobutane pentane cyclopentane hexane cyclohexane heptane cycloheptane octane cyclooctane nonane cyclononane "At -40". bUnder pressure. polycyclic compounds, which have rings with common carbons, and these will be discussed later in this chapter. -

Chemical Communications COMMUNICATION
Please do not adjust margins Chemical Communications COMMUNICATION Multielectron C–H Photoactivation with an Sb(V) Oxo Corrole Christopher M. Lemon,† Andrew G. Maher, Anthony R. Mazzotti, David C. Powers,‡ Miguel I. Received 00th January 20xx, Gonzalez, and Daniel G. Nocera* Accepted 00th January 20xx DOI: 10.1039/x0xx00000x www.rsc.org/ Pnictogen complexes are ideal for mediating multi-electron chemical reactions in two-electron steps. We report a Sb(V) bis-μ- oxo corrole that photochemically oxidises the C–H bonds of organic substrates. In the case of toluene, the substrate is oxidised to benzaldehyde, a rare example of a four-electron photoreaction. Nature utilizes the cytochrome P450 family of enzymes to perform a variety of aerobic oxidations including hydrocarbon 1 hydroxylation, alkene epoxidation, and N- and S-oxidation. Figure 1. Synthesis of the Sb(V) oxo dimer 2 (Ar = C6F5), which can be prepared using Antimony porphyrins have garnered interest as cytochrome various oxidants: H2O2 • urea (74% yield), PhI(OAc)2/O2 (56% yield), or PhIO (71% yield). P450 mimics,2–4 owing to similarities between the absorption5 and magnetic circular dichroism6 spectra of Sb(III) porphyrins As an alternative to manipulating the Sb–X bond by outer- and the CO adduct of cytochrome P450. Augmenting these sphere electron transfer, we have developed a direct halogen spectroscopic relationships, antimony porphyrins mediate a multielectron photochemistry by exploiting the Sb(III)/Sb(V)X2 23 variety of photochemical oxidations7,8 including alkene couple. This approach complements M–X photoactivation at n+2 n… n+2 epoxidation9,10 and hydrocarbon hydroxylation.11,12 These the M X2 centre of two-electron mixed-valence (M M ) 24–27 reactions demonstrate that main group complexes can serve complexes with second- and third-row transition metals. -

Rate Coefficients for Reactions of OH with Aromatic and Aliphatic Volatile Organic Compounds Determined by the Multivariate Relative Rate Technique Jacob T
https://doi.org/10.5194/acp-2020-281 Preprint. Discussion started: 22 April 2020 c Author(s) 2020. CC BY 4.0 License. Rate coefficients for reactions of OH with aromatic and aliphatic volatile organic compounds determined by the Multivariate Relative Rate Technique Jacob T. Shaw1*, Andrew R. Rickard1,2, Mike J. Newland1 and Terry J. Dillon1 5 1 Wolfson Atmospheric Chemistry Laboratories, Department of Chemistry, University of York, Heslington, York, YO10 5DD, UK 2 National Centre for Atmospheric Science, University of York, Heslington, York, YO10 5DD, UK * Now at Department of Earth and Environmental Science, University of Manchester, Manchester, M13 9PL, UK Correspondence to: Terry J. Dillon ([email protected]) 10 1 https://doi.org/10.5194/acp-2020-281 Preprint. Discussion started: 22 April 2020 c Author(s) 2020. CC BY 4.0 License. Abstract. The multivariate relative rate method was applied to a range of volatile organic compounds (VOC) reactions with OH. This previously published method (Shaw et al., 2018b) was improved to increase the sensitivity towards slower reacting VOC, broadening the range of compounds which can be examined. A total of thirty-five room temperature relative rate coefficients were determined; eight of which have not previously been reported. Five of the new reaction rate coefficients were 5 for large alkyl substituted monoaromatic species recently identified in urban air masses, likely with large ozone production -12 3 -1 -1 potentials. The new results (with kOH (296 K) values in units of 10 cm molecule s ) were: n-butylbenzene, 11 (± 4); n- pentylbenzene, 7 (± 2); 1,2-diethylbenzene, 14 (± 4); 1,3-diethylbenzene, 22 (± 4) and 1,4-diethylbenzene, 16 (± 4). -

1 Chapter 3: Organic Compounds: Alkanes and Cycloalkanes
Chapter 3: Organic Compounds: Alkanes and Cycloalkanes >11 million organic compounds which are classified into families according to structure and reactivity Functional Group (FG): group of atoms which are part of a large molecule that have characteristic chemical behavior. FG’s behave similarly in every molecule they are part of. The chemistry of the organic molecule is defined by the function groups it contains 1 C C Alkanes Carbon - Carbon Multiple Bonds Carbon-heteroatom single bonds basic C N C C C X X= F, Cl, Br, I amines Alkenes Alkyl Halide H C C C O C C O Alkynes alcohols ethers acidic H H H C S C C C C S C C H sulfides C C thiols (disulfides) H H Arenes Carbonyl-oxygen double bonds (carbonyls) Carbon-nitrogen multiple bonds acidic basic O O O N H C H C O C Cl imine (Schiff base) aldehyde carboxylic acid acid chloride O O O O C C N C C C C O O C C nitrile (cyano group) ketones ester anhydrides O C N amide opsin Lys-NH2 + Lys- opsin H O H N rhodopsin H 2 Alkanes and Alkane Isomers Alkanes: organic compounds with only C-C and C-H single (s) bonds. general formula for alkanes: CnH(2n+2) Saturated hydrocarbons Hydrocarbons: contains only carbon and hydrogen Saturated" contains only single bonds Isomers: compounds with the same chemical formula, but different arrangement of atoms Constitutional isomer: have different connectivities (not limited to alkanes) C H O C4H10 C5H12 2 6 O OH butanol diethyl ether straight-chain or normal hydrocarbons branched hydrocarbons n-butane n-pentane Systematic Nomenclature (IUPAC System) Prefix-Parent-Suffix -

Studies of Produced Water Toxicity Using Luminescent Marine Bacteria
Environmental Toxicology 111 Studies of produced water toxicity using luminescent marine bacteria S. Grigson, C. Cheong & E. Way Heriot-Watt University, Scotland, UK Abstract The main aqueous discharge from oil production platforms is produced water (PW). Produced water is contaminated with a range of pollutants including crude oil, inorganic salts, trace metals, dissolved gases, produced solids and oilfield chemical residues. Concern has been expressed on the impact these discharges, and particularly the dissolved oil component, may be having on the marine environment. In this investigation the toxicity of synthetic produced waters contaminated with petroleum hydrocarbons was compared to PW samples received from the field using the luminescent marine bacterium Vibrio fisheri. The objective was to correlate toxicity to specific PW components. Initial studies of individual oil components showed that both aromatic and aliphatic compounds exhibited toxicity. Naphthalene was the most toxic aromatic compound measured and cycloheptane the most toxic aliphatic. For benzenes, toxicity increased with alkyl substitution. Synthetic PW samples, based on the composition of those obtained offshore, had lower toxicities than the field PW samples. The addition of oilfield chemicals at dosage levels used offshore increased the toxicity of the synthetic PW mixtures, but not to the original values. Removal of the oil components by solid-phase extraction reduced PW toxicity in both synthetic and real samples. The results suggest that a range of hydrocarbons, both aliphatic and aromatic, along with heavy metals and oilfield chemical residues, contribute to the toxicity of produced water. Removal of petroleum hydrocarbons significantly reduces the acute toxicity of produced water. However, differences in toxicity between real and synthetic PW samples suggest that components other than hydrocarbons, heavy metals and oilfield chemical residues, are also influencing the toxicity of the effluent. -

Organic & Biomolecular Chemistry
Organic & Biomolecular Chemistry Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. We will replace this Accepted Manuscript with the edited and formatted Advance Article as soon as it is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/obc Page 1 of 49 Organic & Biomolecular Chemistry Stable Analogues of Nojirimycin – Synthesis and Biological Evaluation of Nojiristegine and manno -Nojiristegine Agnete H. Viuff,a Louise M. Besenbacher,a Akiko Kamoric, c Mikkel T. Jensen, a Mogens Kilian,b Atsushi Kato c and Henrik H. Jensen* ,a Manuscript aDepartment of Chemistry, Aarhus University, Langelandsgade 140, 8000, Aarhus C, Denmark. bDepartment of Biomedicine, Aarhus University, Wilhelm Meyers allé 4, 8000 Aarhus C, Denmark. cDepartment of Hospital Pharmacy, University of Toyama, 2630 Sugitani, Toyama 930-01940, Japan. Accepted [email protected] Abstract Chemistry Two novel iminosugars called nojiristegines, being structural hybrids between nor-tropane alkaloid calystegine and nojirimycins, have been synthesised and found to be stable molecules despite the presence of a hemiaminal functionality. -

Compound Other Saturated Aliphatic Hydrocarbons C6-C8 Data Collection Sheet (1/4)
Compound Other saturated aliphatic hydrocarbons C6-C8 Data collection sheet (1/4) See Annex 1 for existing human health-based CLP See Table 1 for CAS numbers in the factsheet classifications in the factsheet Organisation name AgBB ANSES BAuA ECHA ECHA DNEL(general population, long-term, inhalation, systemic) DNEL(general population, long-term, [Hydrocarbons, C6-C7, n- NIK (=LCI) CLI (=LCI) OEL (aliphatic C6-C8) inhalation, systemic) alkanes, isoalkanes, (octane & isooctane) cyclics, <5% n-hexane” Risk value name (EC number: 921-024-6)] Risk value (µg/m3) 15000 10000 700000 608000 608000 Risk value (ppb) N/A N/A N/A N/A ~130000 Reference period Chronic Chronic Chronic Chronic Chronic Year 2012 1993 2017 2011, updated 2017 2011, updated in 2017 GLP-guideline study GLP-guideline study (OECD guideline 413) as (OECD guideline 413) as Key study N/A N/A N/A reported in the reported in the registration dossiers for registration dossier octane and isooctane Subchronic inhalation Subchronic inhalation Study type N/A N/A N/A study study Species N/A N/A N/A Rat Rat 6 h/d, 5 d/wk for 13 6 h/d, 5 d/wk for 13 Duration of exposure N/A N/A N/A weeks weeks Value based on provisional The group limit value Subchronic toxicity or Subchronic toxicity or OEL by the German BAuA is based on the 700 neurotoxic effects of neurotoxic effects of Critical effect for C5-C8 aliphatic N/A mg/m³ (200 ppm) “light alkylate naphtha “light alkylate naphtha hydrocarbons in 2012 limit value for distillate” (CAS 64741-66- distillate” (CAS 64741-66- (BAuA, 2012). -

Predicting Sooting Propensity of Oxygenated Fuels Using Artificial
processes Article Predicting Sooting Propensity of Oxygenated Fuels Using Artificial Neural Networks Abdul Gani Abdul Jameel Department of Chemical Engineering, King Fahd University of Petroleum & Minerals, Dhahran 31261, Saudi Arabia; [email protected] Abstract: The self-learning capabilities of artificial neural networks (ANNs) from large datasets have led to their deployment in the prediction of various physical and chemical phenomena. In the present work, an ANN model was developed to predict the yield sooting index (YSI) of oxygenated fuels using the functional group approach. A total of 265 pure compounds comprising six chemical classes, namely paraffins (n and iso), olefins, naphthenes, aromatics, alcohols, and ethers, were dis-assembled into eight constituent functional groups, namely paraffinic CH3 groups, paraffinic CH2 groups, paraffinic CH groups, olefinic –CH=CH2 groups, naphthenic CH-CH2 groups, aromatic C-CH groups, alcoholic OH groups, and ether O groups. These functional groups, in addition to molecular weight and branching index, were used as inputs to develop the ANN model. A neural network with two hidden layers was used to train the model using the Levenberg–Marquardt (ML) training algorithm. The developed model was tested with 15% of the random unseen data points. A regression coefficient (R2) of 0.99 was obtained when the experimental values were compared with the predicted YSI values from the test set. An average error of 3.4% was obtained, which is less than the experimental uncertainty associated with most reported YSI measurements. The developed model can be used for YSI prediction of hydrocarbon fuels containing alcohol and ether-based oxygenates as additives with a high degree of accuracy. -

Approved Minutes for the Division Committee Meeting
Minutes DC 19 Sept 2020 (online) International Union of Pure and Applied Chemistry Division VIII Chemical Nomenclature and Structure Representation Approved Minutes for the Division Committee Meeting Date: Saturday, 19 September 2020 Time: 08:00 EST (05:00 PDT, 13:00 BST, 14:00 CEST, 15:00 EEST, 24:00 NZST) Venue: Online (GoToMeeting platform) 1. Welcome, introductory remarks and housekeeping announcements Alan Hutton (ATH) welcomed everybody to the meeting, extending a special welcome to those who were attending the Division Committee (DC) meeting for the first time. Because of the Covid-19 pandemic, the (online) format of the meeting was different from previous years, and he described the working protocols and arrangements for the meeting. He noted that the time was early in the morning in the USA, midnight in New Zealand, and that the meeting would have to move along swiftly to cover the agenda items that were dealt with during the normal one-and-a-half day physical meeting. 2. Attendance and apologies Present for all or part of the meeting: Alan T. Hutton (President, ATH), Risto S. Laitinen (Secretary, RSL), Michael A. Beckett (MAB), Edwin C. Constable (ECC), Ture Damhus (TD), Safiye Erdem (SE), Adeyinka Fasakin (AF), Richard M. Hartshorn (RMH), Robin Macaluso (RM), Elisabeth Mansfield (EM), Leah R. McEwen (LRM), Gerard P. Moss (GPM), Warren Powell (WP), Amelia P. Rauter (APR), Molly A. Strausbaugh (MAS), Erik Szabó (ES), Augusto Tomé (AT), Clare A. Tovee (CAT), Jiří Vohlídal (JV), Andrey Yerin (AY) (For the Division VIII membership in 2020-2021 and the group photo of the meeting attendees, see Appendix 1) Apologies: Thomas Engel (TE), Steve Heller (SH), Karl-Heinz Hellwich (KHH), Ebbe Nordlander (EN), Michelle M.