Introduction to Organic Chemistry: Hydrocarbons
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Supplementary Information for “Oligomeric Models for Estimation of Polydimethylsiloxane
Electronic Supplementary Material (ESI) for Environmental Science: Processes & Impacts. This journal is © The Royal Society of Chemistry 2016 Supplementary Information for “Oligomeric models for estimation of polydimethylsiloxane- water partition ratios with COSMO-RS theory: Impact of the combinatorial term on absolute error” by J. Mark Parnis and Donald Mackay. Contents: 1) The complete set of chemicals, with the published values of log K(PDMS-w), and the abbreviated reference, corresponding to references given in the principle text. 1) The complete set of chemicals, with the published values of log KPDMS-w. Hsieh 2011 CHAO 2014 PCB18 4.91 PCB203 7.09 PCB16 5.12 PCB195 6.89 PCB32 5.12 PCB194 6.79 PCB28,31 5.17 Benzyl alcohol -0.35 PCB33,53 5.18 4-Fluorophenol -0.28 PCB22 5.30 m-Cresol -0.03 PCB52 5.48 Phenethyl alcohol 0.12 PCB47,48 5.49 3-Methylbenzyl alcohol 0.17 PCB44 5.44 3-Chlorophenol 0.31 PCB71 5.49 3,5-Dimethylphenol 0.42 PCB41 5.49 3-Bromophenol 0.46 PCB70 5.79 4-Ethylphenol 0.60 PCB66 5.70 4-Chloroaniline 0.84 PCB95 5.77 Phenyl acetate 0.86 PCB56,60 5.86 Benzonitrile 1.04 PCB101 6.01 Acetophenone 1.04 PCB99 6.17 4-Chloroacetophenone 1.64 PCB83 6.02 Methyl benzoate 1.65 PCB97 6.05 Benzene 1.76 PCB87 6.19 Ethylbenzoate 2.12 PCB85 6.38 Toluene 2.28 PCB110 6.03 4-Chloroanisole 2.37 PCB151 6.31 Chlorobenzene 2.40 PCB135 6.48 Bromobenzene 2.51 PCB149 6.42 o-Xylene 2.69 PCB118 6.23 m-Xylene 2.73 PCB146 6.66 Iodobenzene 2.73 PCB153 6.62 p-Xylene 2.75 PCB105,132 6.40 Ethylbenzene 2.75 PCB179 6.73 Phenol -0.18 PCB141 6.73 Naphthalene 2.83 PCB138 6.61 Cyclopentane 2.85 PCB163 6.56 4-Chlorotoluene 2.87 PCB158 6.83 Methylcyclopentane 3.13 PCB187 6.96 Propylbenzene 3.14 PCB182 6.96 Isopropylbenzene 3.15 PCB183 6.26 1,3,5-Trimethylbenzene 3.16 PCB128 6.61 1-Methyl-4-Ethylbenzene 3.20 PCB185 6.86 Cyclohexane 3.20 PCB174 7.04 1-Methylnaphthalene 3.26 PCB177 7.02 1,2,4-Trimethylbenzene 3.34 PCB171,202 6.78 tert-Butylbenzene 3.34 PCB180 6.89 Biphenyl 3.37 PCB170 6.82 2,3-Dimethylbutane 3.37 PCB201 7.06 2-Methylpentane 3.51 CHAO 2014 Kang et al. -

Minutes of the IUPAC Chemical Nomenclature and Structure Representation Division (VIII) Committee Meeting Boston, MA, USA, August 18, 2002
Minutes of the IUPAC Chemical Nomenclature and Structure Representation Division (VIII) Committee Meeting Boston, MA, USA, August 18, 2002 Members Present: Dr Stephen Heller, Prof Herbert Kaesz, Prof Dr Alexander Lawson, Prof G. Jeffrey Leigh, Dr Alan McNaught (President), Dr. Gerard Moss, Prof Bruce Novak, Dr Warren Powell (Secretary), Dr William Town, Dr Antony Williams Members Absent: Dr. Michael Dennis, Prof Michael Hess National representatives Present: Prof Roberto de Barros Faria (Brazil) The second meeting of the Division Committee of the IUPAC Division of Chemical Nomenclature and Structure Representation held in the Great Republic Room of the Westin Hotel in Boston, Massachusetts, USA was convened by President Alan McNaught at 9:00 a.m. on Sunday, August 18, 2002. 1.0 President McNaught welcomed the members to this meeting in Boston and offered a special welcome to the National Representative from Brazil, Prof Roberto de Barros Faria. He also noted that Dr Michael Dennis and Prof Michael Hess were unable to be with us. Each of the attendees introduced himself and provided a brief bit of background information. Housekeeping details regarding breaks and lunch were announced and an invitation to a reception from the U. S. National Committee for IUPAC on Tuesday, August 20 was noted. 2.0 The agenda as circulated was approved with the addition of a report from Dr Moss on the activity on his website. 3.0 The minutes of the Division Committee Meeting in Cambridge, UK, January 25, 2002 as posted on the Webboard (http://www.rsc.org/IUPAC8/attachments/MinutesDivCommJan2002.rtf and http://www.rsc.org/IUPAC8/attachments/MinutesDivCommJan2002.pdf) were approved with the following corrections: 3.1 The name Dr Gerard Moss should be added to the members present listing. -

Combined PIANO Standard
Combined PIANO Standard Product #: VHG-PIANO-COM-0.1 Lot #: 711069047B Concentration Concentration Component Component (Wt.%) (Wt.%) Isoparaffins Isopentane 0.3371 4-Methylheptane 0.4939 2,3-Dimethylbutane 0.0691 3-Methylheptane 0.8406 2-Methylpentane 0.5031 3-Ethylhexane 0.1097 3-Methylpentane 0.8271 3,3-Dimethylheptane 0.2590 2,2-Dimethylpentane 0.2746 2,5-Dimethylheptane 0.8678 2,4-Dimethylpentane 0.5679 3,5-Dimethylheptane 0.1169 2,2,3-Trimethylbutane 0.6042 2,3-Dimethylheptane 0.2288 3,3-Dimethylpentane 0.2873 3,4-Dimethylheptane 0.5668 2-Methylhexane 0.3643 2-Methyloctane 0.5791 2,3-Dimethylpentane 0.2751 3-Methyloctane 0.8645 3-Methylhexane 0.2475 3,3-Diethylpentane 0.2424 3-Ethylpentane 0.0813 2,2-Dimethyloctane 0.5024 2,2-Dimethylhexane 0.2027 3,3-Dimethyloctane 0.4902 2,5-Dimethylhexane 0.5727 2,3-Dimethyloctane 0.5927 2,2,3-Trimethylpentane 0.2658 3-Ethyloctane 0.5694 2,4-Dimethylhexane 0.2542 2-Methylnonane 0.5727 2,3-Dimethylhexane 0.2492 3-Methylnonane 0.8889 2-Methylheptane 0.6744 Aromatics Benzene 1.6808 1-Methyl-3-n-propylbenzene 0.4972 Toluene 1.0772 1-Methyl-4-n-propylbenzene 0.5237 Ethylbenzene 1.5918 n-Butylbenzene 0.5198 m-Xylene 0.5341 1,2-Diethylbenzene 0.2577 p-Xylene 1.1328 1-Methyl-2-n-propylbenzene 0.5282 o-Xylene 0.5315 1,4-Dimethyl-2-ethylbenzene 0.5379 Isopropylbenzene 0.5235 1,3-Dimethyl-5-ethylbenzene 0.5176 n-Propylbenzene 1.0731 1,2-Dimethyl-4-ethylbenzene 0.5310 1-Methyl-3-ethylbenzene 0.5181 1,3-Dimethyl-2-ethylbenzene 0.2695 1-Methyl-4-ethylbenzene 0.5104 1,2-Dimethyl-3-ethylbenzene 0.5151 1,3,5-Trimethylbenzene -

Cyclobutane Derivatives in Drug Discovery
Cyclobutane Derivatives in Drug Discovery Overview Key Points Unlike larger and conformationally flexible cycloalkanes, Cyclobutane adopts a rigid cyclobutane and cyclopropane have rigid conformations. Due to the ring strain, cyclobutane adopts a rigid puckered puckered conformation Offer ing advantages on (~30°) conformation. This unique architecture bestowed potency, selectivity and certain cyclobutane-containing drugs with unique pharmacokinetic (PK) properties. When applied appropriately, cyclobutyl profile. scaffolds may offer advantages on potency, selectivity and pharmacokinetic (PK) profile. Bridging Molecules for Innovative Medicines 1 PharmaBlock designs and Cyclobutane-containing Drugs synthesizes over 1846 At least four cyclobutane-containing drugs are currently on the market. cyclobutanes, and 497 Chemotherapy carboplatin (Paraplatin, 1) for treating ovarian cancer was cyclobutane products are prepared to lower the strong nephrotoxicity associated with cisplatin. By in stock. CLICK HERE to replacing cisplatin’s two chlorine atoms with cyclobutane-1,1-dicarboxylic find detailed product acid, carboplatin (1) has a much lower nephrotoxicity than cisplatin. On information on webpage. the other hand, Schering-Plough/Merck’s hepatitis C virus (HCV) NS3/4A protease inhibitor boceprevir (Victrelis, 2) also contains a cyclobutane group in its P1 region. It is 3- and 19-fold more potent than the 1 corresponding cyclopropyl and cyclopentyl analogues, respectively. Androgen receptor (AR) antagonist apalutamide (Erleada, 4) for treating castration-resistant prostate cancer (CRPC) has a spirocyclic cyclobutane scaffold. It is in the same series as enzalutamide (Xtandi, 3) discovered by Jung’s group at UCLA in the 2000s. The cyclobutyl- (4) and cyclopentyl- derivative have activities comparable to the dimethyl analogue although the corresponding six-, seven-, and eight-membered rings are slightly less 2 active. -

Recent Advances in the Total Synthesis of Cyclobutane-Containing Natural Products Cite This: Org
Volume 7 | Number 1 | 7 January 2020 ORGANIC CHEMISTRY FRONTIERS rsc.li/frontiers-organic ORGANIC CHEMISTRY FRONTIERS View Article Online REVIEW View Journal | View Issue Recent advances in the total synthesis of cyclobutane-containing natural products Cite this: Org. Chem. Front., 2020, 7, 136 Jinshan Li,†a Kai Gao, †a Ming Bianb and Hanfeng Ding *a,c Complex natural products bearing strained cyclobutane subunits, including terpenoids, alkaloids and steroids, not only display fascinating architectures, but also show potent biological activities. Due to their unique structures as critical core skeletons in these molecules, a variety of new strategies for the con- Received 24th September 2019, struction of cyclobutane rings have greatly emerged during the last decade. In this review, we wish to Accepted 11th November 2019 summarize the recent progress in the cyclobutane-containing natural product synthesis with an emphasis DOI: 10.1039/c9qo01178a on disconnection tactics employed to forge the four-membered rings, aiming to provide a complement rsc.li/frontiers-organic to existing reviews. 1. Introduction stereoselectively, poses significant challenges in synthetic chemistry. On the other hand, cyclobutanes readily undergo a In the class of strained carbocycles, cyclobutanes have been number of ring-opening reactions by virtue of their tendency known as intriguing structural motifs for more than one to release inherent strain energies. In some cases, however, century but remained relatively less explored in parallel with striking ring strains can be dramatically reduced by the instal- their homologues.1 Due to the highly strained ring systems (ca. lation of a gem-dialkyl substituent (through the Thorpe–Ingold − 26.7 kcal mol 1), construction of cyclobutane rings, especially effect),2 a carbonyl group, a heteroatom, or other functional- ities (Fig. -

Third-Generation Synchrotron X-Ray Diffraction of 6- M Crystal of Raite, Na
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 12263–12267, November 1997 Geology Third-generation synchrotron x-ray diffraction of 6-mm crystal of raite, 'Na3Mn3Ti0.25Si8O20(OH)2z10H2O, opens up new chemistry and physics of low-temperature minerals (crystal structureymicrocrystalyphyllosilicate) JOSEPH J. PLUTH*, JOSEPH V. SMITH*†,DMITRY Y. PUSHCHAROVSKY‡,EUGENII I. SEMENOV§,ANDREAS BRAM¶, CHRISTIAN RIEKEL¶,HANS-PETER WEBER¶, AND ROBERT W. BROACHi *Department of Geophysical Sciences, Center for Advanced Radiation Sources, GeologicalySoilyEnvironmental, and Materials Research Science and Engineering Center, 5734 South Ellis Avenue, University of Chicago, Chicago, IL 60637; ‡Department of Geology, Moscow State University, Moscow, 119899, Russia; §Fersman Mineralogical Museum, Russian Academy of Sciences, Moscow, 117071, Russia; ¶European Synchrotron Radiation Facility, BP 220, 38043, Grenoble, France; and UOP Research Center, Des Plaines, IL 60017 Contributed by Joseph V. Smith, September 3, 1997 ABSTRACT The crystal structure of raite was solved and the energy and metal industries, hydrology, and geobiology. refined from data collected at Beamline Insertion Device 13 at Raite lies in the chemical cooling sequence of exotic hyperal- the European Synchrotron Radiation Facility, using a 3 3 3 3 kaline rocks of the Kola Peninsula, Russia, and the 65 mm single crystal. The refined lattice constants of the Monteregian Hills, Canada (2). This hydrated sodium- monoclinic unit cell are a 5 15.1(1) Å; b 5 17.6(1) Å; c 5 manganese silicate extends the already wide range of manga- 5.290(4) Å; b 5 100.5(2)°; space group C2ym. The structure, nese crystal chemistry (3), which includes various complex including all reflections, refined to a final R 5 0.07. -

Chapter 2: Alkanes Alkanes from Carbon and Hydrogen
Chapter 2: Alkanes Alkanes from Carbon and Hydrogen •Alkanes are carbon compounds that contain only single bonds. •The simplest alkanes are hydrocarbons – compounds that contain only carbon and hydrogen. •Hydrocarbons are used mainly as fuels, solvents and lubricants: H H H H H H H H H H H H C H C C H C C C C H H C C C C C H H H C C H H H H H H CH2 H CH3 H H H H CH3 # of carbons boiling point range Use 1-4 <20 °C fuel (gasses such as methane, propane, butane) 5-6 30-60 solvents (petroleum ether) 6-7 60-90 solvents (ligroin) 6-12 85-200 fuel (gasoline) 12-15 200-300 fuel (kerosene) 15-18 300-400 fuel (heating oil) 16-24 >400 lubricating oil, asphalt Hydrocarbons Formula Prefix Suffix Name Structure H CH4 meth- -ane methane H C H H C H eth- -ane ethane 2 6 H3C CH3 C3H8 prop- -ane propane C4H10 but- -ane butane C5H12 pent- -ane pentane C6H14 hex- -ane hexane C7H16 hept- -ane heptane C8H18 oct- -ane octane C9H20 non- -ane nonane C10H22 dec- -ane decane Hydrocarbons Formula Prefix Suffix Name Structure H CH4 meth- -ane methane H C H H H H C2H6 eth- -ane ethane H C C H H H H C H prop- -ane propane 3 8 H3C C CH3 or H H H C H 4 10 but- -ane butane H3C C C CH3 or H H H C H 4 10 but- -ane butane? H3C C CH3 or CH3 HydHrydorcocaarrbobnos ns Formula Prefix Suffix Name Structure H CH4 meth- -ane methane H C H H H H C2H6 eth- -ane ethane H C C H H H H C3H8 prop- -ane propane H3C C CH3 or H H H C H 4 10 but- -ane butane H3C C C CH3 or H H H C H 4 10 but- -ane iso-butane H3C C CH3 or CH3 HydHrydoroccarbrobnsons Formula Prefix Suffix Name Structure H H -

11.2 Alkanes
11.2 Alkanes A large number of carbon compounds are possible because the covalent bond between carbon atoms, such as those in hexane, C6H14, are very strong. Learning Goal Write the IUPAC names and draw the condensed structural formulas and skeletal formulas for alkanes and cycloalkanes. Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition © 2015 Pearson Education, Inc. Naming Alkanes Alkanes • are hydrocarbons that contain only C—C and C—H bonds • are formed by a continuous chain of carbon atoms • are named using the IUPAC (International Union of Pure and Applied Chemistry) system • have names that end in ane • use Greek prefixes to name carbon chains with five or more carbon atoms Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition © 2015 Pearson Education, Inc. IUPAC Naming of First Ten Alkanes Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition © 2015 Pearson Education, Inc. 1 Condensed Structural Formulas In a condensed structural formula, • each carbon atom and its attached hydrogen atoms are written as a group • a subscript indicates the number of hydrogen atoms bonded to each carbon atom The condensed structural formula of butane has four carbon atoms. CH3—CH2—CH2—CH3 butane Core Chemistry Skill Naming and Drawing Alkanes Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition © 2015 Pearson Education, Inc. Condensed Structural Formulas Alkanes are written with structural formulas that are • expanded to show each bond • condensed to show each carbon atom and its attached hydrogen atoms Expanded Condensed Expanded Condensed Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition © 2015 Pearson Education, Inc. -
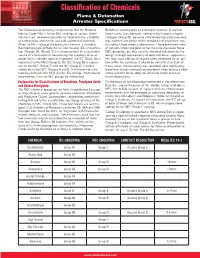
Classification of Chemicals
Classification of Chemicals Flame & Detonation Arrester Specifications PROTECTOSEAL ® The Protectoseal Company recommends that the National Butadiene would qualify as a Group D material. In each of Electric Code (NEC) Article 500, rankings of various chemi - these cases, the chemicals were primarly listed in a higher cals be used, whenever possible, to determine the suitability category (Group B), because of relatively high pressure read - of a detonation arrester for use with a particular chemical. ings noted in one phase of the standard test procedure con - When no NEC rating of the particular chemical is available, ducted by Underwriters Laboratories. These pressures were the International Electrotechnical Commission (IEC) classifica - of concern when categorizing the chemicals because these tion (Groups IIA, IIB and IIC) is recommended as a secondary NEC groupings are also used as standard indicators for the source of information for determining the suitability of an ar - design strength requirements of electrical boxes, apparatus, rester for its intended service. In general, the IEC Group IIA is etc. that must withstand the pressures generated by an igni - equivalent to the NEC Group D; the IEC Group IIB is equiva - tion within the container. It should be noted that, in each of lent to the NEC Group C; and the IEC Group IIC includes these cases, the test pressures recorded were significantly chemicals in the NEC Groups A and B. In the event of a dis - lower than those commonly encountered when testing a deto - crepancy between the NEC and the IEC ratings, Protectoseal nation arrester for its ability to withstand stable and over - recommends that the NEC groups be referenced. -

Cycloalkanes, Cycloalkenes, and Cycloalkynes
CYCLOALKANES, CYCLOALKENES, AND CYCLOALKYNES any important hydrocarbons, known as cycloalkanes, contain rings of carbon atoms linked together by single bonds. The simple cycloalkanes of formula (CH,), make up a particularly important homologous series in which the chemical properties change in a much more dramatic way with increasing n than do those of the acyclic hydrocarbons CH,(CH,),,-,H. The cyclo- alkanes with small rings (n = 3-6) are of special interest in exhibiting chemical properties intermediate between those of alkanes and alkenes. In this chapter we will show how this behavior can be explained in terms of angle strain and steric hindrance, concepts that have been introduced previously and will be used with increasing frequency as we proceed further. We also discuss the conformations of cycloalkanes, especially cyclo- hexane, in detail because of their importance to the chemistry of many kinds of naturally occurring organic compounds. Some attention also will be paid to polycyclic compounds, substances with more than one ring, and to cyclo- alkenes and cycloalkynes. 12-1 NOMENCLATURE AND PHYSICAL PROPERTIES OF CYCLOALKANES The IUPAC system for naming cycloalkanes and cycloalkenes was presented in some detail in Sections 3-2 and 3-3, and you may wish to review that ma- terial before proceeding further. Additional procedures are required for naming 446 12 Cycloalkanes, Cycloalkenes, and Cycloalkynes Table 12-1 Physical Properties of Alkanes and Cycloalkanes Density, Compounds Bp, "C Mp, "C diO,g ml-' propane cyclopropane butane cyclobutane pentane cyclopentane hexane cyclohexane heptane cycloheptane octane cyclooctane nonane cyclononane "At -40". bUnder pressure. polycyclic compounds, which have rings with common carbons, and these will be discussed later in this chapter. -

Process for the Preparation of (11Α,13E,15S)-11,15-Dihydroxy-9-Oxoprost-13-En-1-Oic Acid
Technical Disclosure Commons Defensive Publications Series July 2020 Process for the preparation of (11α,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en-1-oic acid Srinivasan Thirumalai Rajan Follow this and additional works at: https://www.tdcommons.org/dpubs_series Recommended Citation Srinivasan Thirumalai Rajan, "Process for the preparation of (11α,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en-1-oic acid", Technical Disclosure Commons, (July 10, 2020) https://www.tdcommons.org/dpubs_series/3418 This work is licensed under a Creative Commons Attribution 4.0 License. This Article is brought to you for free and open access by Technical Disclosure Commons. It has been accepted for inclusion in Defensive Publications Series by an authorized administrator of Technical Disclosure Commons. : Process for the preparation of (11?,13E,15S)-11,15-dihydroxy-9-ox Process for the preparation of (11α,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en- 1-oic acid Field of the invention: 5 The present application relates a process for the preparation of (11α,13E,15S)- 11,15-dihydroxy-9-oxoprost-13-en-1-oic acid. Formula-1 10 Background of the invention: (11α,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en-1-oic acid is generally known as Prostaglandin E1 (PGE1) or Alprostadil. Alprostadil was approved in US and Europe under the brand name of CAVERJECT® and indicated for the treatment 15 of erectile dysfunction due to neurogenic, vasculogenic, psychogenic, or mixed etiology. Biosynthetic PGE1, is formed from dihomo-γ-linolenic acid was disclosed in Prostaglandin 20, 187, 1980. Syntheses of PGE1 was disclosed in J.