Cyclohexene Oxide 286-20-4 NTP NOMINATION HISTORY and REVIEW
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Synthesis of Polycyclic Natural Products Jianmin Shi Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1990 Synthesis of polycyclic natural products Jianmin Shi Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Shi, Jianmin, "Synthesis of polycyclic natural products " (1990). Retrospective Theses and Dissertations. 9891. https://lib.dr.iastate.edu/rtd/9891 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. JLÎMI MICROFILMED 1991 INFORMATION TO USERS The most advanced technology has been used to photograph and reproduce this manuscript from the microfihn master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. -

Cyclohexane Oxidation Continues to Be a Challenge Ulf Schuchardt A,∗, Dilson Cardoso B, Ricardo Sercheli C, Ricardo Pereira A, Rosenira S
Applied Catalysis A: General 211 (2001) 1–17 Review Cyclohexane oxidation continues to be a challenge Ulf Schuchardt a,∗, Dilson Cardoso b, Ricardo Sercheli c, Ricardo Pereira a, Rosenira S. da Cruz d, Mário C. Guerreiro e, Dalmo Mandelli f , Estevam V. Spinacé g, Emerson L. Pires a a Instituto de Qu´ımica, Universidade Estadual de Campinas, P.O. Box 6154, 13083-970 Campinas, SP, Brazil b Depto de Eng. Qu´ımica, Universidade Federal de São Carlos, 13565-905 São Carlos, SP, Brazil c College of Chemistry, University of California, Berkeley, CA 94720, USA d Depto Ciências Exatas e Tecnológicas, Universidade Estadual de Santa Cruz, 45650-000 Ilhéus, BA, Brazil e Universidade Federal de Lavras, Lavras, MG, Brazil f Instituto de Ciências Biológicas e Qu´ımicas, PUC-Campinas, 13020-904 Campinas, SP, Brazil g Sup. Caracterização Qu´ımica, IPEN, 05508-900 São Paulo, SP, Brazil Received 3 October 2000; received in revised form 21 December 2000; accepted 28 December 2000 Abstract Many efforts have been made to develop new catalysts to oxidize cyclohexane under mild conditions. Herein, we review the most interesting systems for this process with different oxidants such as hydrogen peroxide, tert-butyl hydroperoxide and molecular oxygen. Using H2O2, Na-GeX has been shown to be a most stable and active catalyst. Mesoporous TS-1 and Ti-MCM-41 are also stable, but the use of other metals such as Cr, V, Fe and Mo leads to leaching of the metal. Homogeneous systems based on binuclear manganese(IV) complexes have also been shown to be interesting. When t-BuOOH is used, the active systems are those phthalocyanines based on Ru, Co and Cu and polyoxometalates of dinuclear ruthenium and palladium. -

POLYMERISATION of SOME CYCLIC ETHERS and ALLYL COMPOUNDS at Higf PRESSURES
POLYMERISATION OF SOME CYCLIC ETHERS AND ALLYL COMPOUNDS AT HIGf PRESSURES. THESIS SUBMITTED FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN THE FACULTY OF SCIENCE UNIVERSITY OF LONDON BY MUSTAFIZUR RAHMAN. DEPARTMENT OF CHEMICAL ENGINEERING AND CHEMICAL TECHNOLOGY, IMPERIAL COLLEGE OF SCIENCE AND TECHNOLOGY, LONDON, S.W.7. SEPTEMBER, 1967. ABSTRACT. The polymerizations of seven cyclic ethers and two allyl compounds have been studied at high pressures (up to 12,000 atm.). BF3.(C2H5)20 was usually used as catalyst to polymerize the ethers, except for 1,4-epoxycyclohexane with wilich the co-catalyst epichlorohydrin was employed. Benzoyl peroxide and azo-isobutyronitrile were used to polymerize the allyl compounds. The polymerizations of 1,4-epoxycyclohexane and tetrahydrofuran (THF) have been studied most extensively. From the lag rate vs. pressure graphs, "overall volumes of activation" Air*pol were calculated and compared with those for vinyl compounds. Conductances of solutions of BP3.(C2H5)20 in tetrahydropyran were measured and the results discussed in relation to the kinetic measurements. The variation of the polymerization ceiling temperature of THF with pressure was determined between 1 and 2500 atm. The polymerizations of cyclohexene oxide, styrene cxide, cyclooctene oxide, tetrahydropyran, triallyl phosphate, triallyl phosphite and the co-polymerization of triallyl phosphate and styrene, have been studied briefly. The results, wherever possible, have been explained in terms of existing ideas. 3. Polymers from viscous liquid to insoluble solids were obtained during this investigation and the molecular weights were determined, where possible. 4 ACKNOWLEDGEBENTS. The author wishes to express his gratitude to Dr. K.E.Weale for his kind supervision, valuane guidance and constant encouragement during the course of this study. -

Perfluoroalkyl-Iodo Norbornane Compounds and Their Use As
Europâisches Patentamt (g) ÛJ)) European Patent Office (0) Publication number: O 002 74 1 Office européen des brevets Bl © EUROPEAN PATENT SPECIFICATION @ Date of publication of patent spécification: 18.11.81 @© Int.lnt.CI.3: Cl.3: C 07 C 61/12, C07C 103/19, number: 78101696.9 @ Application CC07C 07 C 103/737,103/737 @ Date offiling: 15.12.78 C 07 D 209/76, C07B 29/04, C07C 69/74, C 07 D 307/77, B 01 F 17/00 (54) Perfluoroaikyl-iodo norbornane compounds and their use as surfactants. (§) Priority: 27.12.77 US 865060 @ Proprietor: CIBA-GEIGY AG Patentabteilung Postfach CH-4002 Basel (CH) (43) Date of publication of application: 11.07.79 Bulletin 79/14 (72) Inventor: Brace, Neal O. 1 022 East North Path (45) Publication of the grant of the European patent: Wheaton Illinois 60187 (US) 18.11.81 Bulletin 81/46 (84) Designated Contracting States: CH DE FR GB (56) References cited: NL - A - 68 08078 Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. Notice of opposition shall be filed in a written reasoned statement. It shall not be deemed to have been filed until the opposition fee has been paid. (Art. 99(1 ) European patent convention). Courier Press, Leamington Spa, England. The compounds of this invention are perfluoroalkyliodo norbornane derivatives of the formula wherein Y is independently oxygen or the group >NR, R is independently hydrogen or alkyl of 1 to 24 carbon atoms or each group - YR is independently halogen with the proviso that if two halogen atoms are present these are identical and Rf is a straight or branched chain perfluoroalkyl of 1 to 18 carbon atoms or said perfluoroalkyl substituted by a perfluoroalkoxy group of 2 to 6 carbon atoms. -

Revised Group Additivity Values for Enthalpies of Formation (At 298 K) of Carbon– Hydrogen and Carbon–Hydrogen–Oxygen Compounds
Revised Group Additivity Values for Enthalpies of Formation (at 298 K) of Carbon– Hydrogen and Carbon–Hydrogen–Oxygen Compounds Cite as: Journal of Physical and Chemical Reference Data 25, 1411 (1996); https://doi.org/10.1063/1.555988 Submitted: 17 January 1996 . Published Online: 15 October 2009 N. Cohen ARTICLES YOU MAY BE INTERESTED IN Additivity Rules for the Estimation of Molecular Properties. Thermodynamic Properties The Journal of Chemical Physics 29, 546 (1958); https://doi.org/10.1063/1.1744539 Critical Evaluation of Thermochemical Properties of C1–C4 Species: Updated Group- Contributions to Estimate Thermochemical Properties Journal of Physical and Chemical Reference Data 44, 013101 (2015); https:// doi.org/10.1063/1.4902535 Estimation of the Thermodynamic Properties of Hydrocarbons at 298.15 K Journal of Physical and Chemical Reference Data 17, 1637 (1988); https:// doi.org/10.1063/1.555814 Journal of Physical and Chemical Reference Data 25, 1411 (1996); https://doi.org/10.1063/1.555988 25, 1411 © 1996 American Institute of Physics for the National Institute of Standards and Technology. Revised Group Additivity Values for Enthalpies of Formation (at 298 K) of Carbon-Hydrogen and Carbon-Hydrogen-Oxygen Compounds N. Cohen Thermochemical Kinetics Research, 6507 SE 31st Avenue, Portland, Oregon 97202-8627 Received January 17, 1996; revised manuscript received September 4, 1996 A program has been undertaken for the evaluation and revision of group additivity values (GAVs) necessary for predicting, by means of Benson's group additivity method, thermochemical properties of organic molecules. This review reports on the portion of that program dealing with GAVs for enthalpies of formation at 298.15 K (hereinafter abbreviated as 298 K) for carbon-hydrogen and carbon-hydrogen-oxygen compounds. -
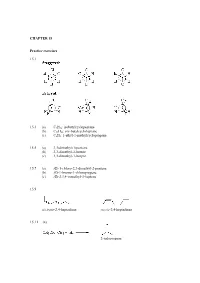
CHAPTER 15 Practice Exercises 15.1 15.3 (A) C9H18
CHAPTER 15 Practice exercises 15.1 15.3 (a) C9H18: isobutylcyclopentane (b) C11H22: sec-butylcycloheptane (c) C6H2: 1-ethyl-1-methylcyclopropane 15.5 (a) 3,3-dimethyl-1-pentene (b) 2,3-dimethyl-2-butene (c) 3,3-dimethyl-1-butyne 15.7 (a) (E)-1-chloro-2,3-dimethyl-2-pentene (b) (Z)-1-bromo-1-chloropropene (c) (E)-2,3,4-trimethyl-3-heptene 15.9 cis,trans-2,4-heptadiene cis,cis-2,4-heptadiene 15.11 (a) 2-iodopropane (b) 1-iodo-1-methylcyclohexane 15.13 CH3 CH3 + HI I Step 1: Protonation of the alkene to give the most stable 3˚ carbocation intermediate: slow step + CH3 HI CH3 I rate-determining step Step 2: Nucleophilic attack of the iodide anion on the 3˚ carbocation intermediate to give the product: CH + 3 CH3 I I 15.15 H2O CH3 CH3 + H3O OH Step 1: Protonation of the alkene to give the most stable 3˚ carbocation intermediate: slow step H + + CH3 HOH CH3 O H rate-determining H step Step 2: Nucleophilic attack of the water on the 3˚ carbocation intermediate to give the protonated alcohol: H H O+ H + CH3 O H CH3 Step 3: The protonated alcohol loses a proton to form the product: H + O H H CH3 + O + H3O CH3 H OH 15.17 (a) 2-phenyl-2-propanol (b) (E)-3,4-diphenyl-3-hexene (c) 3-methylbenzoic acid or m-methylbenzoic acid Review questions 15.1 A hydrocarbon is a compound composed only of hydrogen and carbon atoms. 15.3 In saturated hydrocarbons, each carbon is bonded to four other atoms, either hydrogen or carbon atoms. -

In This Handout, All of Our Functional Groups Are Presented As Condensed Line Formulas, 2D and 3D Formulas and with Nomenclature Prefixes and Suffixes (If Present)
In this handout, all of our functional groups are presented as condensed line formulas, 2D and 3D formulas and with nomenclature prefixes and suffixes (if present). Organic names are built on a foundation of alkanes, alkenes and alkynes. Those examples are presented first and you need to know those rules. The strategies can be found in Chapter 4 of our textbook (alkanes: pages 93-98, cycloalkanes 102-104, alkenes: pages 104-110, alkynes: pages 112-113 and combinations of all of them 113-115). After introducing examples of alkanes, alkenes, alkynes and combinations of them, the functional groups are presented in order of priority. A few nomenclature examples are provided for each of the functional groups. Examples of the various functional groups are presented on pages 115-135 in the textbook. Two overview pages are on pages 136-137. Some functional groups have a suffix name when they are the highest priority functional group and a prefix name when they are not the highest priority group, and these are added to the skeletal names with identifying numbers and stereochemistry terms (E and Z for alkenes, R and S for chiral centers and cis and trans for rings). Several low priority functional groups only have a prefix name. A few additional special patterns are shown on pages 98-102. The only way to learn this topic is practice (over and over). The best practice approach is to actually write out the names (on an extra piece of paper or on a white board, and then do it again). The same functional groups are used throughout the entire course. -

Chem 314 Preorganic Evaluation
Organic Reaction Guide Beauchamp 1 Chem 316 / Beauchamp Reactions Review Sheet Name SN2 Reactions - special features: biomolecular kinetics Rate = kSN2[RX][Nu ], single step concerted reaction, E2 is a competing reaction o o o o relative order of reactivity: CH3X > 1 RX > 2 RX >> 3 RX (based on steric hinderance, no SN2 at 3 RX) allylic & benzylic RX are very reactive, adjacent pi bonds help stabilize transition state and lower TS energy (Ea) o complete substitution at Cα (3 RX) or Cβ (neopentyl pattern) almost completely inhibits SN2 reactions vinyl & phenyl are very unreactive, bonds are stronger and poor backside approach leaving group ability: OTs = I > Br > Cl in neutral or basic conditions (just like E2, SN1 adn E1), and neutral molecule leaving groups are good from protonated, cationic intermediates in acid conditions, + + + + -OH2 , -ORH , -OR2 , -NR3 , etc. we will consider all anions, ammonia, amines, thiols and sulfides to be strong nucleophiles (favors SN2 and E2 reactions) in our course some electron pair donors are mainly nucleophiles (sulfur, azide, cyanide, carboxylates) and - + + - + - some are mainly bases (t-BuO K , Na H2N , Na H ) polar, aprotic solvents work best for SN2 reactions because nucleophiles are relatively unencombered for electron doantion (dimethyl sulofoxide = DMSO, dimethylformamide = DMF, acetonitrile = AN, acetone, etc.) in our course some electron pair donors are mainly nucleophiles (sulfur, azide, cyanide, carboxylates) and we will consider neutral solvent molecules such as water, alcohols and acids to be weak nucleophiles (favors SN1 and E1) stereoselectivity: 100% inversion of configuration from backside atack regioselectivity: reacts at carbon with leaving group, completely unambiguous chemoselectivity: N/A The following list is designed to emphasize SN2 reactions. -

NXP Semiconductors NXP List of Hazardous Substances in Products
NXP Semiconductors NX3-00119 NXP List of Hazardous Substances Sustainability 01-October-2012 in Products and Packaging Sustainability Page 1 of 7 Supersedes: NX3-00119 01-October-2010 Table of contents 1 Objectives ............................................................................................................................. 2 2 Scope ................................................................................................................................... 2 3 Responsibilities, Records & Risks......................................................................................... 2 3.1 Roles and Responsibilities .................................................................................................... 2 3.2 Records ................................................................................................................................ 2 3.3 Risks .................................................................................................................................... 3 4 References ........................................................................................................................... 3 5 Definitions/Abbreviations ...................................................................................................... 3 5.1 Definitions ............................................................................................................................. 3 5.2 Abbreviations ....................................................................................................................... -

The Photochemistry of Some Substituted 2-Cyclohexenones and the Excited States Involved
PHOTOCHEMISTRY OF SUBSTITUTi':D 2... CYCLOHE:XE;NONES THE PHOTOCHEMISTRY OF S0~1E SUBSTITUTED 2~CYCLOHEXENONES AND THE EXCITED STATES INVOLVED By FLOlTI FP~ERICK SNYDER, B.Sce .A Thesis Submitted to the Faculty of Graduate Studies in Partial Fulfilment of the Requirements for the Degree Master of Science McMaster University October 1969 To MOM and DAD on Your 25th Anniversary MASTER OF SCIENCE (1969) McMASTER UNIVERSITY (Ghemi stry) Hamilton, Ontario. TITLE: The Photochemistry of some Substituted 2-Cyclohexenones and the Excited States Involved AUTHOR: Floyd Frederick Snyder, B.Sc. '(University of Alberta) SUPERVISOR: Dr. J. J. McCullough NUMBER OF PAGES: viii, 87 SCOPE AND CONTENTS: The photoadditions of 3~phenyl-2-cyclohexenone to bicyclo [2.2.1] hepta-2,5-diene, bicyclo [2.2.1] hept-2mene and cyclopentene have been studied. In all cases £.!1! fused cyclobutane products were obtained. Quenching and sensitization experiments indicated a singlet excited state to be active in photocycloaddition. Phosphorescence and fluorescence emission were observed from 3-phenyl-2-cyclohexenoneo Energy transfer to the lo~.rest triplet of 3-phenyl-2-cyclohexenone was evident from the quenching of Michler's ketone phosphorescence. Two norbornene dimers were detected in the photolysis of 3-phenyl-2-cyclo hexenone and norbornene giving evidence for a higher triplet excited state of the enone. The photoaddition of 3-methyl-2-cyclohexenone to cyclopentene l.ras studied for comparison and both .£!..! and !.r..!.U! fused adducts l-Tere obtained. In photolyses with bicyclo [2e2el] hepta-2,5 diene or cyclopentene, 2-phenyl... 2-cyclohexenone lvas unreactive. iii ACKNOWLEDGEMENTS It is a pleasure to express my gratitude to Dr. -

Cyclohexene Oxide/Carbon Dioxide Copolymerization by Chromium(III) Amino-Bis(Phenolato) Complexes and MALDI- TOF MS Analysis of the Polycarbonates
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is © The Royal Society of Chemistry 2015 Electronic Supplementary Information for Cyclohexene Oxide/Carbon Dioxide Copolymerization by Chromium(III) Amino-bis(phenolato) Complexes and MALDI- TOF MS Analysis of the Polycarbonates Katalin Devaine-Pressing,† Louise N. Dawe,†,‡ and Christopher M. Kozak*,† † Department of Chemistry, Memorial University of Newfoundland, St. John’s, Newfoundland, A1B 3X7, Canada. ‡ C-CART X-ray Diffraction Laboratory, Department of Chemistry, Memorial University of Newfoundland, St. John’s, Newfoundland, A1B 3X7, Canada. Current address: Department of Chemistry and Biochemistry, Wilfrid Laurier University, 75 University Ave. W. Waterloo, Ontario, N2L 3C5, Canada Experimental S3 Table S1. Crystallographic and structure refinement data for 1·THF, 1·DMAP and 2·THF S10 Figure S1. MALDI-TOF mass spectrum of 1·THF S11 Figure S2. MALDI-TOF mass spectrum of 1·THF higher mass region. S11 Figure S3. MALDI-TOF mass spectrum of 2·THF S12 Figure S4. MALDI-TOF mass spectrum of 1·DMAP S13 Figure S5. UV-Vis absorption spectrum of 1·THF S14 Figure S6. UV-Vis absorption spectrum of 2·THF S14 Figure S7. UV-Vis absorption spectrum of 1·DMAP S15 Figure S8. IR spectrum of 1·THF S16 Figure S9. IR spectrum of 2·THF S16 Figure S10. IR spectrum of 1·DMAP S17 Figure S11. The first 20 min of the reaction profile of the growth of the polycarbonate ν(C=O) BuBuPy catalyzed by 1·THF, 1·DMAP and CrCl[O2NN’] S17 Figure S12. The second stage of the propagation of the reaction catalyzed by BuBuPy CrCl[O2NN’] . -

THE EFFECTS of SEVERAL PAPER CHARACTERISTICS and APPLICATION METHODS on the SUBLIMATION RATE of CYCLODODECANE by Kelli A. Piot
THE EFFECTS OF SEVERAL PAPER CHARACTERISTICS AND APPLICATION METHODS ON THE SUBLIMATION RATE OF CYCLODODECANE by Kelli A. Piotrowski A thesis submitted to the Department of Art In conformity with the requirements for the degree of Master of Art Conservation Queen’s University Kingston, Ontario, Canada (September, 2013) Copyright © Kelli Piotrowski, 2013 ABSTRACT Cyclododecane (CDD) is a waxy, solid cyclic hydrocarbon (C12H24) that sublimes at room temperature and possesses strong hydrophobicity. In paper conservation CDD is used principally as a temporary fixative of water-soluble media during aqueous treatments. Hydrophobicity, ease of reversibility, low toxicity, and absence of residues are reasons often cited for its use over alternative materials although the latter two claims continue to be debated in the literature. The sublimation rate has important implications for treatment planning as well as health and safety considerations given the dearth of reliable information on its toxicity and exposure limits. This study examined how the rate of sublimation is affected by fiber type, sizing, and surface finish as well as delivery in the molten phase and as a saturated solution in low boiling petroleum ether. The effect of warming the paper prior to application was also evaluated. Sublimation was monitored using gravimetric analysis after which samples were tested for residues with gas chromatography-flame ionization detection (GC-FID) to confirm complete sublimation. Water absorbency tests were conducted to determine whether this property is fully reestablished. Results suggested that the sublimation rate of CDD is affected minimally by all of the paper characteristics and application methods examined in this study. The main factors influencing the rate appear to be the thickness and mass of the CDD over a given surface area as well as temperature and ventilation.