7.2 Conformations of Cyclohexane 269
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Implications for Extraterrestrial Hydrocarbon Chemistry: Analysis Of
The Astrophysical Journal, 889:3 (26pp), 2020 January 20 https://doi.org/10.3847/1538-4357/ab616c © 2020. The American Astronomical Society. All rights reserved. Implications for Extraterrestrial Hydrocarbon Chemistry: Analysis of Acetylene (C2H2) and D2-acetylene (C2D2) Ices Exposed to Ionizing Radiation via Ultraviolet–Visible Spectroscopy, Infrared Spectroscopy, and Reflectron Time-of-flight Mass Spectrometry Matthew J. Abplanalp1,2 and Ralf I. Kaiser1,2 1 W. M. Keck Research Laboratory in Astrochemistry, University of Hawaii at Manoa, Honolulu, HI 96822, USA 2 Department of Chemistry, University of Hawaii at Manoa, Honolulu, HI 96822, USA Received 2019 October 4; revised 2019 December 7; accepted 2019 December 10; published 2020 January 20 Abstract The processing of the simple hydrocarbon ice, acetylene (C2H2/C2D2), via energetic electrons, thus simulating the processes in the track of galactic cosmic-ray particles penetrating solid matter, was carried out in an ultrahigh vacuum surface apparatus. The chemical evolution of the ices was monitored online and in situ utilizing Fourier transform infrared spectroscopy (FTIR) and ultraviolet–visible spectroscopy and, during temperature programmed desorption, via a quadrupole mass spectrometer with an electron impact ionization source (EI-QMS) and a reflectron time-of-flight mass spectrometer utilizing single-photon photoionization (SPI-ReTOF-MS) along with resonance-enhanced multiphoton photoionization (REMPI-ReTOF-MS). The confirmation of previous in situ studies of ethylene ice irradiation -

First Principles Prediction of Thermodynamic Properties
2 First Principles Prediction of Thermodynamic Properties Hélio F. Dos Santos and Wagner B. De Almeida NEQC: Núcleo de Estudos em Química Computacional, Departamento de Química, ICE Universidade Federal de Juiz de Fora (UFJF), Campus Universitário Martelos, Juiz de Fora LQC-MM: Laboratório de Química Computacional e Modelagem Molecular Departamento de Química, ICEx, Universidade Federal de Minas Gerais (UFMG) Campus Universitário, Pampulha, Belo Horizonte Brazil 1. Introduction The determination of the molecular structure is undoubtedly an important issue in chemistry. The knowledge of the tridimensional structure allows the understanding and prediction of the chemical-physics properties and the potential applications of the resulting material. Nevertheless, even for a pure substance, the structure and measured properties reflect the behavior of many distinct geometries (conformers) averaged by the Boltzmann distribution. In general, for flexible molecules, several conformers can be found and the analysis of the physical and chemical properties of these isomers is known as conformational analysis (Eliel, 1965). In most of the cases, the conformational processes are associated with small rotational barriers around single bonds, and this fact often leads to mixtures, in which many conformations may exist in equilibrium (Franklin & Feltkamp, 1965). Therefore, the determination of temperature-dependent conformational population is very much welcomed in conformational analysis studies carried out by both experimentalists and theoreticians. -

Text Related to Segment 5.02 ©2002 Claude E. Wintner from the Previous Segment We Have the Value of the Heat of Combustion Of
Text Related to Segment 5.02 ©2002 Claude E. Wintner From the previous segment we have the value of the heat of combustion of an "unstrained" methylene unit as -157.4 kcal/mole. On this basis one would predict that combustion of "unstrained" cyclopropane should liberate 3 X 157.4 = 472.2 kcal/mole; however, when cyclopropane is burned, a value of 499.8 kcal/mole is measured for the actual heat of combustion. The difference of 27.6 kcal/mole is interpreted as representing the higher internal energy ("strain energy") of real cyclopropane as compared to a postulated strain-free "model." ("Model" in this usage speaks of a proposal, or postulate.) These facts are graphed conveniently on an energy diagram as follows: "Strain Energy" represents the error units: kcal/mole not to scale in the "strain-free model" real cyclopropane + 4.5 O2 27.6 "strain-free model" of cyclopropane 499.8 observed heat of combustion + 4.5 O2 472.2 3CO2 + 3H2O heat of combustion predicted for "strain-free model" Note that this estimate of the strain energy in cyclopropane amounts to 9.2 kcal/mole of strain per methylene group (dividing 27.6 by 3, because of the three carbon atoms). Comparable values in kcal/mole for other small and medium rings are given in the following table. As one might expect, in general the strain decreases as the ring is enlarged. units: kcal/mole n Total Strain Strain per CH2 3 27.6 9.2 (CH2)n 4 26.3 6.6 5 6.2 1.2 6 0.1 0.0 ! 7 6.2 0.9 8 9.7 1.2 9 12.6 1.4 10 12.4 1.2 12 4.1 0.3 15 1.9 0.1 Without entering into a discussion of the relevant bonding concepts here, and instead relying on geometry alone, interpretation of the source of the strain energy in cyclopropane and cyclobutane is to some extent self-evident. -

New Chemistry of Donor-Acceptor Cycloalkanes and Studies Towards the Synthesis of Streptorubin B
Western University Scholarship@Western Electronic Thesis and Dissertation Repository 8-2-2016 12:00 AM New Chemistry of Donor-Acceptor Cycloalkanes and Studies Towards the Synthesis of Streptorubin B Naresh Vemula The University of Western Ontario Supervisor Prof. Brian L. Pagenkopf The University of Western Ontario Graduate Program in Chemistry A thesis submitted in partial fulfillment of the equirr ements for the degree in Doctor of Philosophy © Naresh Vemula 2016 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Organic Chemistry Commons Recommended Citation Vemula, Naresh, "New Chemistry of Donor-Acceptor Cycloalkanes and Studies Towards the Synthesis of Streptorubin B" (2016). Electronic Thesis and Dissertation Repository. 3895. https://ir.lib.uwo.ca/etd/3895 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. Abstract and Keywords Abstract This dissertation presents two separate chapters within the broad area of synthetic organic chemistry. The first chapter describes the annelation chemistry of donor-acceptor (DA) cyclopropanes and cyclobutanes for the synthesis of heterocycles. The Yb(OTf)3-catalyzed [4+2] cycloaddition between DA cyclobutanes and nitrosoarenes facilitated the synthesis of tetrahydro-1,2-oxazines in good to excellent yields as single diastereomers. Additionally, an unexpected deoxygenation occurred with electron-rich nitrosoarenes under MgI2-catalysis that afforded pyrrolidine products. The GaCl3-catalyzed [4+2] cycloaddition of DA cyclobutanes and cis-diazenes provided hexahydropyridazine derivatives in good to excellent yields as single diastereomers. -

Toxicological Assessment of Hanford Tank Headspace Chemicals - Determination of Chemicals of Potential Concern
PNNL-14949 Rev. 0 Pacific Northwest National Laboratory Operated by Battelle for the U.S. Department of Energy Toxicological Assessment of Hanford Tank Headspace Chemicals - Determination of Chemicals of Potential Concern I. E. Burgeson N. A. Moore A. L. Bunn J. L. Huckaby December 2004 • Prepared for CH2M HILL Hanford Group, Inc. LEGAL NOTICE This report was prepared by Battelle Memorial Institute (Battelle) as an account of sponsored research activities. Neither Client nor Battelle nor any person acting on behalf of either: MAKES ANY WARRANTY OR REPRESENTATION, EXPRESS OR IMPLIED, with respect to the accuracy, completeness, or usefulness of the information contained in this report, or that the use of any information, apparatus, process, or composition disclosed in this report may not infringe privately owned rights; or Assumes any liabilities with respect to the use of, or for damages resulting from the use of, any information, apparatus, process, or composition disclosed in this report. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by Battelle. The views and opinions of authors expressed herein do not necessarily state or reflect those of Battelle. @ This document was printed on recycled paper. (9/97) PNNL-14949 Rev. 0 Toxicological Assessment of Hanford Tank Headspace Chemicals - Determination of Chemicals of Potential Concern I. E. Burgeson N. A. Moore A. L. Bunn J. L. Huckaby December 2004 Prepared for CH2M HILL Hanford Group, Inc. Pacific Northwest Nationa] Laboratory Richland, Washington 99352 Summary A toxicological assessment of chemicals found, or predicted to be present, in Hanford tank headspaces was performed by Pacific Northwest National Laboratory in support of the Industrial Hygiene Chemical Vapor Technical Basis produced by CH2M HILL Hanford Group, Inc. -

Cycloalkanes, Cycloalkenes, and Cycloalkynes
CYCLOALKANES, CYCLOALKENES, AND CYCLOALKYNES any important hydrocarbons, known as cycloalkanes, contain rings of carbon atoms linked together by single bonds. The simple cycloalkanes of formula (CH,), make up a particularly important homologous series in which the chemical properties change in a much more dramatic way with increasing n than do those of the acyclic hydrocarbons CH,(CH,),,-,H. The cyclo- alkanes with small rings (n = 3-6) are of special interest in exhibiting chemical properties intermediate between those of alkanes and alkenes. In this chapter we will show how this behavior can be explained in terms of angle strain and steric hindrance, concepts that have been introduced previously and will be used with increasing frequency as we proceed further. We also discuss the conformations of cycloalkanes, especially cyclo- hexane, in detail because of their importance to the chemistry of many kinds of naturally occurring organic compounds. Some attention also will be paid to polycyclic compounds, substances with more than one ring, and to cyclo- alkenes and cycloalkynes. 12-1 NOMENCLATURE AND PHYSICAL PROPERTIES OF CYCLOALKANES The IUPAC system for naming cycloalkanes and cycloalkenes was presented in some detail in Sections 3-2 and 3-3, and you may wish to review that ma- terial before proceeding further. Additional procedures are required for naming 446 12 Cycloalkanes, Cycloalkenes, and Cycloalkynes Table 12-1 Physical Properties of Alkanes and Cycloalkanes Density, Compounds Bp, "C Mp, "C diO,g ml-' propane cyclopropane butane cyclobutane pentane cyclopentane hexane cyclohexane heptane cycloheptane octane cyclooctane nonane cyclononane "At -40". bUnder pressure. polycyclic compounds, which have rings with common carbons, and these will be discussed later in this chapter. -

Revised Group Additivity Values for Enthalpies of Formation (At 298 K) of Carbon– Hydrogen and Carbon–Hydrogen–Oxygen Compounds
Revised Group Additivity Values for Enthalpies of Formation (at 298 K) of Carbon– Hydrogen and Carbon–Hydrogen–Oxygen Compounds Cite as: Journal of Physical and Chemical Reference Data 25, 1411 (1996); https://doi.org/10.1063/1.555988 Submitted: 17 January 1996 . Published Online: 15 October 2009 N. Cohen ARTICLES YOU MAY BE INTERESTED IN Additivity Rules for the Estimation of Molecular Properties. Thermodynamic Properties The Journal of Chemical Physics 29, 546 (1958); https://doi.org/10.1063/1.1744539 Critical Evaluation of Thermochemical Properties of C1–C4 Species: Updated Group- Contributions to Estimate Thermochemical Properties Journal of Physical and Chemical Reference Data 44, 013101 (2015); https:// doi.org/10.1063/1.4902535 Estimation of the Thermodynamic Properties of Hydrocarbons at 298.15 K Journal of Physical and Chemical Reference Data 17, 1637 (1988); https:// doi.org/10.1063/1.555814 Journal of Physical and Chemical Reference Data 25, 1411 (1996); https://doi.org/10.1063/1.555988 25, 1411 © 1996 American Institute of Physics for the National Institute of Standards and Technology. Revised Group Additivity Values for Enthalpies of Formation (at 298 K) of Carbon-Hydrogen and Carbon-Hydrogen-Oxygen Compounds N. Cohen Thermochemical Kinetics Research, 6507 SE 31st Avenue, Portland, Oregon 97202-8627 Received January 17, 1996; revised manuscript received September 4, 1996 A program has been undertaken for the evaluation and revision of group additivity values (GAVs) necessary for predicting, by means of Benson's group additivity method, thermochemical properties of organic molecules. This review reports on the portion of that program dealing with GAVs for enthalpies of formation at 298.15 K (hereinafter abbreviated as 298 K) for carbon-hydrogen and carbon-hydrogen-oxygen compounds. -
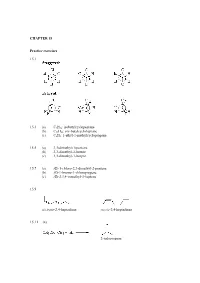
CHAPTER 15 Practice Exercises 15.1 15.3 (A) C9H18
CHAPTER 15 Practice exercises 15.1 15.3 (a) C9H18: isobutylcyclopentane (b) C11H22: sec-butylcycloheptane (c) C6H2: 1-ethyl-1-methylcyclopropane 15.5 (a) 3,3-dimethyl-1-pentene (b) 2,3-dimethyl-2-butene (c) 3,3-dimethyl-1-butyne 15.7 (a) (E)-1-chloro-2,3-dimethyl-2-pentene (b) (Z)-1-bromo-1-chloropropene (c) (E)-2,3,4-trimethyl-3-heptene 15.9 cis,trans-2,4-heptadiene cis,cis-2,4-heptadiene 15.11 (a) 2-iodopropane (b) 1-iodo-1-methylcyclohexane 15.13 CH3 CH3 + HI I Step 1: Protonation of the alkene to give the most stable 3˚ carbocation intermediate: slow step + CH3 HI CH3 I rate-determining step Step 2: Nucleophilic attack of the iodide anion on the 3˚ carbocation intermediate to give the product: CH + 3 CH3 I I 15.15 H2O CH3 CH3 + H3O OH Step 1: Protonation of the alkene to give the most stable 3˚ carbocation intermediate: slow step H + + CH3 HOH CH3 O H rate-determining H step Step 2: Nucleophilic attack of the water on the 3˚ carbocation intermediate to give the protonated alcohol: H H O+ H + CH3 O H CH3 Step 3: The protonated alcohol loses a proton to form the product: H + O H H CH3 + O + H3O CH3 H OH 15.17 (a) 2-phenyl-2-propanol (b) (E)-3,4-diphenyl-3-hexene (c) 3-methylbenzoic acid or m-methylbenzoic acid Review questions 15.1 A hydrocarbon is a compound composed only of hydrogen and carbon atoms. 15.3 In saturated hydrocarbons, each carbon is bonded to four other atoms, either hydrogen or carbon atoms. -

In This Handout, All of Our Functional Groups Are Presented As Condensed Line Formulas, 2D and 3D Formulas and with Nomenclature Prefixes and Suffixes (If Present)
In this handout, all of our functional groups are presented as condensed line formulas, 2D and 3D formulas and with nomenclature prefixes and suffixes (if present). Organic names are built on a foundation of alkanes, alkenes and alkynes. Those examples are presented first and you need to know those rules. The strategies can be found in Chapter 4 of our textbook (alkanes: pages 93-98, cycloalkanes 102-104, alkenes: pages 104-110, alkynes: pages 112-113 and combinations of all of them 113-115). After introducing examples of alkanes, alkenes, alkynes and combinations of them, the functional groups are presented in order of priority. A few nomenclature examples are provided for each of the functional groups. Examples of the various functional groups are presented on pages 115-135 in the textbook. Two overview pages are on pages 136-137. Some functional groups have a suffix name when they are the highest priority functional group and a prefix name when they are not the highest priority group, and these are added to the skeletal names with identifying numbers and stereochemistry terms (E and Z for alkenes, R and S for chiral centers and cis and trans for rings). Several low priority functional groups only have a prefix name. A few additional special patterns are shown on pages 98-102. The only way to learn this topic is practice (over and over). The best practice approach is to actually write out the names (on an extra piece of paper or on a white board, and then do it again). The same functional groups are used throughout the entire course. -

Conversion of Waste Plastic Into Liquid Hydrocarbons (ENERGY) by Cuco 3 Catalyst: Application of Scientific Research on Plastic Pollution
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by International Institute for Science, Technology and Education (IISTE): E-Journals Chemical and Process Engineering Research www.iiste.org ISSN 2224-7467 (Paper) ISSN 2225-0913 (Online) Vol.48, 2017 Conversion of Waste Plastic into Liquid Hydrocarbons (ENERGY) by Cuco 3 Catalyst: Application of Scientific Research on Plastic Pollution Manvir Singh 1 Sudesh Kumar 1 Moinuddin Sarker 2 1.Department of Chemistry Banasthali University Rajasthan india 2.Waste Technologies LLC, Department of Research & Development, 1376 Chopsey Hill Road, Bridgeport, CT- 06606, USA Abstract Waste plastics were converted into valuable liquid hydrocarbon fuel. it is can be used as different purpose of energy-source such as petrol engines, diesel engines, generators, vehicles and its good source of chemicals etc. Plastics have many properties like light weight, high durability so its demand increases in every sector. Pyrolysis of the waste plastic (hdpe) was carried out with CuCO 3 catalysts and temperature range from 0 °C to 390 °C. The collected liquid hydrocarbons fuel was characterized by FT-IR, NMR, GCxGCMS spectrometer and fuel density was 78 g ml -1 and conversion was very good. The research paper exhibits concentrating on application for solving daily life issues and problems of plastic. Keywords: Pyrolysis, Liquid hydrocarbons fuel, CuCO 3 catalysts, Conversion, Glass reactor, GCxGCMS. Introduction First time plastic was invented by Alexander Parkes in 1862 that has a high molecular weight (Brydson 1999). A molecule formed by repetition of simple units is called polymer. Plastic is also called polymer for example polyethylene (Chanda 2000). -

Industrial Hydrocarbon Processes
Handbook of INDUSTRIAL HYDROCARBON PROCESSES JAMES G. SPEIGHT PhD, DSc AMSTERDAM • BOSTON • HEIDELBERG • LONDON NEW YORK • OXFORD • PARIS • SAN DIEGO SAN FRANCISCO • SINGAPORE • SYDNEY • TOKYO Gulf Professional Publishing is an imprint of Elsevier Gulf Professional Publishing is an imprint of Elsevier The Boulevard, Langford Lane, Kidlington, Oxford OX5 1GB, UK 30 Corporate Drive, Suite 400, Burlington, MA 01803, USA First edition 2011 Copyright Ó 2011 Elsevier Inc. All rights reserved No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means electronic, mechanical, photocopying, recording or otherwise without the prior written permission of the publisher Permissions may be sought directly from Elsevier’s Science & Technology Rights Department in Oxford, UK: phone (+44) (0) 1865 843830; fax (+44) (0) 1865 853333; email: [email protected]. Alternatively you can submit your request online by visiting the Elsevier web site at http://elsevier.com/locate/ permissions, and selecting Obtaining permission to use Elsevier material Notice No responsibility is assumed by the publisher for any injury and/or damage to persons or property as a matter of products liability, negligence or otherwise, or from any use or operation of any methods, products, instructions or ideas contained in the material herein. Because of rapid advances in the medical sciences, in particular, independent verification of diagnoses and drug dosages should be made British Library Cataloguing in Publication Data -

1 Chapter 3: Organic Compounds: Alkanes and Cycloalkanes
Chapter 3: Organic Compounds: Alkanes and Cycloalkanes >11 million organic compounds which are classified into families according to structure and reactivity Functional Group (FG): group of atoms which are part of a large molecule that have characteristic chemical behavior. FG’s behave similarly in every molecule they are part of. The chemistry of the organic molecule is defined by the function groups it contains 1 C C Alkanes Carbon - Carbon Multiple Bonds Carbon-heteroatom single bonds basic C N C C C X X= F, Cl, Br, I amines Alkenes Alkyl Halide H C C C O C C O Alkynes alcohols ethers acidic H H H C S C C C C S C C H sulfides C C thiols (disulfides) H H Arenes Carbonyl-oxygen double bonds (carbonyls) Carbon-nitrogen multiple bonds acidic basic O O O N H C H C O C Cl imine (Schiff base) aldehyde carboxylic acid acid chloride O O O O C C N C C C C O O C C nitrile (cyano group) ketones ester anhydrides O C N amide opsin Lys-NH2 + Lys- opsin H O H N rhodopsin H 2 Alkanes and Alkane Isomers Alkanes: organic compounds with only C-C and C-H single (s) bonds. general formula for alkanes: CnH(2n+2) Saturated hydrocarbons Hydrocarbons: contains only carbon and hydrogen Saturated" contains only single bonds Isomers: compounds with the same chemical formula, but different arrangement of atoms Constitutional isomer: have different connectivities (not limited to alkanes) C H O C4H10 C5H12 2 6 O OH butanol diethyl ether straight-chain or normal hydrocarbons branched hydrocarbons n-butane n-pentane Systematic Nomenclature (IUPAC System) Prefix-Parent-Suffix