Method for Producing Oxidation Product of Cycloalkane
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![C9-14 Aliphatic [2-25% Aromatic] Hydrocarbon Solvents Category SIAP](https://docslib.b-cdn.net/cover/2852/c9-14-aliphatic-2-25-aromatic-hydrocarbon-solvents-category-siap-12852.webp)
C9-14 Aliphatic [2-25% Aromatic] Hydrocarbon Solvents Category SIAP
CoCAM 2, 17-19 April 2012 BIAC/ICCA SIDS INITIAL ASSESSMENT PROFILE Chemical C -C Aliphatic [2-25% aromatic] Hydrocarbon Solvents Category Category 9 14 Substance Name CAS Number Stoddard solvent 8052-41-3 Chemical Names Kerosine, petroleum, hydrodesulfurized 64742-81-0 and CAS Naphtha, petroleum, hydrodesulfurized heavy 64742-82-1 Registry Solvent naphtha, petroleum, medium aliphatic 64742-88-7 Numbers Note: Substances in this category are also commonly known as mineral spirits, white spirits, or Stoddard solvent. CAS Number Chemical Description † 8052-41-3 Includes C8 to C14 branched, linear, and cyclic paraffins and aromatics (6 to 18%), <50ppmV benzene † 64742-81-0 Includes C9 to C14 branched, linear, and cyclic paraffins and aromatics (10 to Structural 25%), <100 ppmV benzene Formula † and CAS 64742-82-1 Includes C8 to C13 branched, linear, and cyclic paraffins and aromatics (15 to 25%), <100 ppmV benzene Registry † Numbers 64742-88-7 Includes C8 to C13 branched, linear, and cyclic paraffins and aromatics (14 to 20%), <50 ppmV benzene Individual category member substances are comprised of aliphatic hydrocarbon molecules whose carbon numbers range between C9 and C14; approximately 80% of the aliphatic constituents for a given substance fall within the C9-C14 carbon range and <100 ppmV benzene. In some instances, the carbon range of a test substance is more precisely defined in the test protocol. In these instances, the specific carbon range (e.g. C8-C10, C9-C10, etc.) will be specified in the SIAP. * It should be noted that other substances defined by the same CAS RNs may have boiling ranges outside the range of 143-254° C and that these substances are not covered by the category. -

Heterocyclic Chemistry Updated
1 | Page Heterocyclic Chemistry By Dr Vipul Kataria Definition: A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements (N, O, S, P) as members of its ring. Introduction: Heterocyclic compounds may be defined as cyclic compounds having as ring members atoms of at least two different elements. It means the cyclic compound has one another atom different than carbon. Heterocyclic compounds have much importance in organic chemistry because of abundant presence of heterocyclic compounds in present pharmaceutical drugs. Like other organic compounds, there are several defined rules for naming heterocyclic compounds. IUPAC rules for nomenclature of heterocyclic systems (SPU 2012, 2 Marks) The system for nomenclature for heterocyclic systems was given by Hantzsch and Widman. The Hantzsch-Widman nomenclature is based on the type (Z) of the heteroatom; the ring size (n) and nature of the ring, whether it is saturated or unsaturated. This system of nomenclature applies to monocyclic 3-to-10-membered ring heterocycles. Type of the heteroatom: The type of heteroatom is indicated by a prefix as shown below for common hetreroatoms. Dr Vipul Kataria, Chemistry Department, V. P. & R. P. T. P. Science College, VV Nagar 2 | Pag e When two or more of the same heteroatoms are present, the prrefix di, tri, tetra… are used. e.g. dioxa, triaza, dithia. If the heteroatoms are different their atomic number in that grroup is considered. Thus order of numbering will be O, S, N, P, Si. Ring size: The ring size is indicated by a suffix according to Table I below. -

Condensed Cyclic Compound, Composition Including The
(19) TZZ¥_ _T (11) EP 3 184 522 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 28.06.2017 Bulletin 2017/26 C07D 403/14 (2006.01) H01L 51/00 (2006.01) (21) Application number: 16205894.5 (22) Date of filing: 21.12.2016 (84) Designated Contracting States: • INAYAMA, Satoshi AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Yokohama-city, Kanagawa 230-0027 (JP) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • ISHII, Norihito PL PT RO RS SE SI SK SM TR Yokohama-city, Kanagawa 230-0027 (JP) Designated Extension States: • SHIBATA, Katsunori BA ME Yokohama-city, Kanagawa 230-0027 (JP) Designated Validation States: • ITO, Mitsunori MA MD Yokohama-city, Kanagawa 230-0027 (JP) (30) Priority: 22.12.2015 JP 2015250531 (74) Representative: Scheuermann, Erik Elkington and Fife LLP (71) Applicant: Samsung Electronics Co., Ltd. Prospect House Gyeonggi-do 16677 (KR) 8 Pembroke Road Sevenoaks, Kent TN13 1XR (GB) (72) Inventors: • KATO, Fumiaki Yokohama-city, Kanagawa 230-0027 (JP) (54) CONDENSED CYCLIC COMPOUND, COMPOSITION INCLUDING THE CONDENSED CYCLIC COMPOUND, ORGANIC LIGHT-EMITTING DEVICE INCLUDING THE CONDENSED CYCLIC COMPOUND, AND METHOD OF MANUFACTURING THE ORGANIC LIGHT-EMITTING DEVICE (57) A condensed cyclic compound, a composition including the condensed cyclic compound, an organic light-emit- ting device including the condensed cyclic compound, and a method of manufacturing the organic light-emitting device are provided. EP 3 184 522 A1 Printed by Jouve, 75001 PARIS (FR) EP 3 184 522 A1 Description FIELD OF THE INVENTION 5 [0001] One or more embodiments relate to a condensed cyclic compound, a composition including the condensed cyclic compound, an organic light-emitting device including the condensed cyclic compound, and a method of manufac- turing the organic light-emitting device. -

Organic Chemistry PEIMS Code: N1120027 Abbreviation: ORGCHEM Number of Credits That May Be Earned: 1/2-1
Course: Organic Chemistry PEIMS Code: N1120027 Abbreviation: ORGCHEM Number of credits that may be earned: 1/2-1 Brief description of the course (150 words or less): Organic chemistry is an introductory course that is designed for the student who intends to continue future study in the sciences. The student will learn the concepts and applications of organic chemistry. Topics covered include aliphatic and aromatic compounds, alcohols, aldehydes, ketones, acids, ethers, amines, spectra, and stereochemistry. A brief introduction into biochemistry is also provided. The laboratory experiments will familiarize the student with the important laboratory techniques, specifically spectroscopy. Traditional high school chemistry courses focus on the inorganic aspects of chemistry whereas organic chemistry introduces the student to organic compounds and their properties, mechanisms of formations, and introduces the student to laboratory techniques beyond the traditional high school chemistry curriculum. Essential Knowledge and Skills of the course: (a) General requirements. This course is recommended for students in Grades 11-12. The recommended prerequisite for this course is AP Chemistry. (b) Introduction. Organic chemistry is designed to introduce students to the fundamental concepts of organic chemistry and key experimental evidence and data, which support these concepts. Students will learn to apply these data and concepts to chemical problem solving. Additionally, students will learn that organic chemistry is still evolving by reading about current breakthroughs in the field. Finally, students will gain appreciation for role that organic chemistry plays in modern technological developments in diverse fields, ranging from biology to materials science. (c) Knowledge and skills. (1) The student will be able to write both common an IUPAC names for the hydrocarbon. -

AROMATIC COMPOUNDS Aromaticity
AROMATICITY AROMATIC COMPOUNDS Aromaticity Benzene - C6H6 H H H H H H H H H H H H Kekulé and the Structure of Benzene Kekule benzene: two forms are in rapid equilibrium 154 pm 134 pm • All bonds are 140 pm (intermediate between C-C and C=C) • C–C–C bond angles are 120° • Structure is planar, hexagonal A Resonance Picture of Bonding in Benzene resonance hybrid 6 -electron delocalized over 6 carbon atoms The Stability of Benzene Aromaticity: cyclic conjugated organic compounds such as benzene, exhibit special stability due to resonance delocalization of -electrons. Heats of hydrogenation + H2 + 120 KJ/mol + 2 H2 + 230 KJ/mol calc'd value= 240 KJ/mol 10 KJ/mol added stability + 3 H 2 + 208 KJ/mol calc'd value= 360 KJ/mol 152 KJ/mol added stability + 3 H2 + 337 KJ/mol 1,3,5-Hexatriene - conjugated but not cyclic Resonance energy of benzene is 129 - 152 KJ/mol An Orbital Hybridization View of Bonding in Benzene • Benzene is a planar, hexagonal cyclic hydrocarbon • The C–C–C bond angles are 120° = sp2 hybridized • Each carbon possesses an unhybridized p-orbital, which makes up the conjugated -system. • The six -electrons are delocalized through the -system The Molecular Orbitals of Benzene - the aromatic system of benzene consists of six p-orbitals (atomic orbitals). Benzene must have six molecular orbitals. Y6 Y4 Y5 six p-orbitals Y2 Y3 Y1 Degenerate orbitals: Y : zero nodes orbitals that have the 1 Bonding same energy Y2 and Y3: one node Y and Y : two nodes 4 5 Anti-bonding Y6: three node Substituted Derivatives of Benzene and Their Nomenclature -

Cycloalkanes, Cycloalkenes, and Cycloalkynes
CYCLOALKANES, CYCLOALKENES, AND CYCLOALKYNES any important hydrocarbons, known as cycloalkanes, contain rings of carbon atoms linked together by single bonds. The simple cycloalkanes of formula (CH,), make up a particularly important homologous series in which the chemical properties change in a much more dramatic way with increasing n than do those of the acyclic hydrocarbons CH,(CH,),,-,H. The cyclo- alkanes with small rings (n = 3-6) are of special interest in exhibiting chemical properties intermediate between those of alkanes and alkenes. In this chapter we will show how this behavior can be explained in terms of angle strain and steric hindrance, concepts that have been introduced previously and will be used with increasing frequency as we proceed further. We also discuss the conformations of cycloalkanes, especially cyclo- hexane, in detail because of their importance to the chemistry of many kinds of naturally occurring organic compounds. Some attention also will be paid to polycyclic compounds, substances with more than one ring, and to cyclo- alkenes and cycloalkynes. 12-1 NOMENCLATURE AND PHYSICAL PROPERTIES OF CYCLOALKANES The IUPAC system for naming cycloalkanes and cycloalkenes was presented in some detail in Sections 3-2 and 3-3, and you may wish to review that ma- terial before proceeding further. Additional procedures are required for naming 446 12 Cycloalkanes, Cycloalkenes, and Cycloalkynes Table 12-1 Physical Properties of Alkanes and Cycloalkanes Density, Compounds Bp, "C Mp, "C diO,g ml-' propane cyclopropane butane cyclobutane pentane cyclopentane hexane cyclohexane heptane cycloheptane octane cyclooctane nonane cyclononane "At -40". bUnder pressure. polycyclic compounds, which have rings with common carbons, and these will be discussed later in this chapter. -

Structure of Benzene, Orbital Picture, Resonance in Benzene, Aromatic Characters, Huckel’S Rule B
Dr.Mrityunjay Banerjee, IPT, Salipur B PHARM 3rd SEMESTER BP301T PHARMACEUTICAL ORGANIC CHEMISTRY –II UNIT I UNIT I Benzene and its derivatives 10 Hours A. Analytical, synthetic and other evidences in the derivation of structure of benzene, Orbital picture, resonance in benzene, aromatic characters, Huckel’s rule B. Reactions of benzene - nitration, sulphonation, halogenationreactivity, Friedelcrafts alkylation- reactivity, limitations, Friedelcrafts acylation. C. Substituents, effect of substituents on reactivity and orientation of mono substituted benzene compounds towards electrophilic substitution reaction D. Structure and uses of DDT, Saccharin, BHC and Chloramine Benzene and its Derivatives Chemists have found it useful to divide all organic compounds into two broad classes: aliphatic compounds and aromatic compounds. The original meanings of the words "aliphatic" (fatty) and "aromatic" (fragrant/ pleasant smell). Aromatic compounds are benzene and compounds that resemble benzene in chemical behavior. Aromatic properties are those properties of benzene that distinguish it from aliphatic hydrocarbons. Benzene: A liquid that smells like gasoline Boils at 80°C & Freezes at 5.5°C It was formerly used to decaffeinate coffee and component of many consumer products, such as paint strippers, rubber cements, and home dry-cleaning spot removers. A precursor in the production of plastics (such as Styrofoam and nylon), drugs, detergents, synthetic rubber, pesticides, and dyes. It is used as a solvent in cleaning and maintaining printing equipment and for adhesives such as those used to attach soles to shoes. Benzene is a natural constituent of petroleum products, but because it is a known carcinogen, its use as an additive in gasoline is now limited. In 1970s it was associated with leukemia deaths. -

Introduction to Alkenes and Alkynes in an Alkane, All Covalent Bonds
Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene, however, only three σ bonds are formed from the alkene carbon -the carbon thus adopts an sp2 hybridization Ethene (common name ethylene) has a molecular formula of CH2CH2 Each carbon is sp2 hybridized with a σ bond to two hydrogens and the other carbon Hybridized orbital allows stronger bonds due to more overlap H H C C H H Structure of Ethylene In addition to the σ framework of ethylene, each carbon has an atomic p orbital not used in hybridization The two p orbitals (each with one electron) overlap to form a π bond (p bonds are not symmetric about the internuclear axis) π bonds are not as strong as σ bonds (in ethylene, the σ bond is ~90 Kcal/mol and the π bond is ~66 Kcal/mol) Thus while σ bonds are stable and very few reactions occur with C-C bonds, π bonds are much more reactive and many reactions occur with C=C π bonds Nomenclature of Alkenes August Wilhelm Hofmann’s attempt for systematic hydrocarbon nomenclature (1866) Attempted to use a systematic name by naming all possible structures with 4 carbons Quartane a alkane C4H10 Quartyl C4H9 Quartene e alkene C4H8 Quartenyl C4H7 Quartine i alkine → alkyne C4H6 Quartinyl C4H5 Quartone o C4H4 Quartonyl C4H3 Quartune u C4H2 Quartunyl C4H1 Wanted to use Quart from the Latin for 4 – this method was not embraced and BUT has remained Used English order of vowels, however, to name the groups -

Biomoleculesbiomolecules
1414Unit Objectives BiomoleculesBiomolecules After studying this Unit, you will be able to • explain the characteristics of “It is the harmonious and synchronous progress of chemical biomolecules like carbohydrates, reactions in body which leads to life”. proteins and nucleic acids and hormones; • classify carbohydrates, proteins, A living system grows, sustains and reproduces itself. nucleic acids and vitamins on the The most amazing thing about a living system is that it basis of their structures; is composed of non-living atoms and molecules. The • explain the difference between pursuit of knowledge of what goes on chemically within DNA and RNA; a living system falls in the domain of biochemistry. Living • describe the role of biomolecules systems are made up of various complex biomolecules in biosystem. like carbohydrates, proteins, nucleic acids, lipids, etc. Proteins and carbohydrates are essential constituents of our food. These biomolecules interact with each other and constitute the molecular logic of life processes. In addition, some simple molecules like vitamins and mineral salts also play an important role in the functions of organisms. Structures and functions of some of these biomolecules are discussed in this Unit. 14.114.114.1 Carbohydrates Carbohydrates are primarily produced by plants and form a very large group of naturally occurring organic compounds. Some common examples of carbohydrates are cane sugar, glucose, starch, etc. Most of them have a general formula, Cx(H2O)y, and were considered as hydrates of carbon from where the name carbohydrate was derived. For example, the molecular formula of glucose (C6H12O6) fits into this general formula, C6(H2O)6. But all the compounds which fit into this formula may not be classified as carbohydrates. -
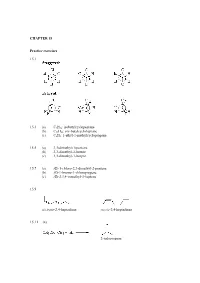
CHAPTER 15 Practice Exercises 15.1 15.3 (A) C9H18
CHAPTER 15 Practice exercises 15.1 15.3 (a) C9H18: isobutylcyclopentane (b) C11H22: sec-butylcycloheptane (c) C6H2: 1-ethyl-1-methylcyclopropane 15.5 (a) 3,3-dimethyl-1-pentene (b) 2,3-dimethyl-2-butene (c) 3,3-dimethyl-1-butyne 15.7 (a) (E)-1-chloro-2,3-dimethyl-2-pentene (b) (Z)-1-bromo-1-chloropropene (c) (E)-2,3,4-trimethyl-3-heptene 15.9 cis,trans-2,4-heptadiene cis,cis-2,4-heptadiene 15.11 (a) 2-iodopropane (b) 1-iodo-1-methylcyclohexane 15.13 CH3 CH3 + HI I Step 1: Protonation of the alkene to give the most stable 3˚ carbocation intermediate: slow step + CH3 HI CH3 I rate-determining step Step 2: Nucleophilic attack of the iodide anion on the 3˚ carbocation intermediate to give the product: CH + 3 CH3 I I 15.15 H2O CH3 CH3 + H3O OH Step 1: Protonation of the alkene to give the most stable 3˚ carbocation intermediate: slow step H + + CH3 HOH CH3 O H rate-determining H step Step 2: Nucleophilic attack of the water on the 3˚ carbocation intermediate to give the protonated alcohol: H H O+ H + CH3 O H CH3 Step 3: The protonated alcohol loses a proton to form the product: H + O H H CH3 + O + H3O CH3 H OH 15.17 (a) 2-phenyl-2-propanol (b) (E)-3,4-diphenyl-3-hexene (c) 3-methylbenzoic acid or m-methylbenzoic acid Review questions 15.1 A hydrocarbon is a compound composed only of hydrogen and carbon atoms. 15.3 In saturated hydrocarbons, each carbon is bonded to four other atoms, either hydrogen or carbon atoms. -

In This Handout, All of Our Functional Groups Are Presented As Condensed Line Formulas, 2D and 3D Formulas and with Nomenclature Prefixes and Suffixes (If Present)
In this handout, all of our functional groups are presented as condensed line formulas, 2D and 3D formulas and with nomenclature prefixes and suffixes (if present). Organic names are built on a foundation of alkanes, alkenes and alkynes. Those examples are presented first and you need to know those rules. The strategies can be found in Chapter 4 of our textbook (alkanes: pages 93-98, cycloalkanes 102-104, alkenes: pages 104-110, alkynes: pages 112-113 and combinations of all of them 113-115). After introducing examples of alkanes, alkenes, alkynes and combinations of them, the functional groups are presented in order of priority. A few nomenclature examples are provided for each of the functional groups. Examples of the various functional groups are presented on pages 115-135 in the textbook. Two overview pages are on pages 136-137. Some functional groups have a suffix name when they are the highest priority functional group and a prefix name when they are not the highest priority group, and these are added to the skeletal names with identifying numbers and stereochemistry terms (E and Z for alkenes, R and S for chiral centers and cis and trans for rings). Several low priority functional groups only have a prefix name. A few additional special patterns are shown on pages 98-102. The only way to learn this topic is practice (over and over). The best practice approach is to actually write out the names (on an extra piece of paper or on a white board, and then do it again). The same functional groups are used throughout the entire course. -

2: Alkanes and Cycloalkanes
(2/94)(1,2,8/95)(6,7/97)(10/98)(1,9-11/99) Neuman Chapter 2 2: Alkanes and Cycloalkanes Alkanes Alkane Systematic Nomenclature Cycloalkanes Conformations of Alkanes Conformations of Cycloalkanes Conformations of Alkylcyclohexanes Preview You learned in the Chapter 1 that all organic molecules have carbon skeletons. These carbon skeletons show great diversity in the ways that C atoms bond to each other, and in their three-dimensional shapes. Alkanes and cycloalkanes consist entirely of carbon skeletons bonded to H atoms since they have no functional groups. As a result, they serve as a basis for understanding the structures of all other organic molecules. This chapter describes the skeleltal isomerism of alkanes and cycloalkanes, their three-dimensional conformations, and their systematic nomenclature that is the basis for the names of all other organic compounds. 2.1 Alkanes We refer to alkanes as hydrocarbons because they contain only C (carbon) and H (hydrogen) atoms. Since alkanes are the major components of petroleum and natural gas, they often serve as a commercial starting point for the preparation of many other classes of organic molecules. Structures of Alkanes (2.1A) Organic chemists use a variety of different types of structures to represent alkanes such as these shown for methane (one C), ethane (two C's), and propane (three C's). [graphic 2.1] Kekulé, Electron-Dot and Three-Dimensional Structures. The structures showing C and H atoms connected by lines are Kekulé structures. Remember from Chapter 1 that these lines represent chemical bonds that are pairs of electrons located in molecular orbitals encompassing the two bonded atoms.